Essentials of Diagnosis
- Following a long asymptomatic period, presentation with heart failure or angina.
- Wide pulse pressure with associated peripheral signs.
- Diastolic decrescendo murmur at the left sternal border.
- Left ventricular dilation and hypertrophy with preserved function.
- Presentation and findings dependent on the rapidity of onset of regurgitation.
- Diagnosis confirmed and severity estimated by Doppler echocardiography, aortography, magnetic resonance imaging, or computed tomography angiography.
Etiology
Normally, the integrity of the aortic orifice during diastole is maintained by an intact aortic root and firm apposition of the free margins of the three aortic valve cusps. Aortic regurgitation (AR) may therefore be caused by a variety of disorders affecting the valve cusps or the aortic root (or both) (Table 18–1). With rheumatic heart disease becoming less common, nonrheumatic causes currently account for most of the underlying causes of AR, including congenitally malformed aortic valves, infective endocarditis, and connective tissue diseases. Disorders affecting the aortic root also account for a large number of patients with AR. These conditions include cystic medial necrosis, Marfan syndrome, aortic dissection, and inflammatory diseases. Even in the absence of any obvious abnormality of the aortic valve or root, severe systemic hypertension has been reported to cause significant AR.
Aortic cusp abnormalities |
Infectious: Bacterial endocarditis, rheumatic fever |
Congenital: Bicuspid aortic valve, Marfan syndrome |
Inflammatory: Systemic lupus erythematosus, rheumatoid arthritis, Behçet syndrome |
Degenerative: Myxomatous (floppy) valve, calcific aortic valve |
Trauma |
Postaortic valvuloplasty |
Diet drug valvulopathy |
Aortic root abnormalities |
Aortic root dilatation: Marfan syndrome, syphilis, ankylosing spondylitis, relapsing polychondritis, idiopathic aortitis, annuloaortic ectasia, cystic medial necrosis, Ehlers-Danlos syndrome |
Loss of commissural support: Aortic dissection, trauma, ventricular septal defect |
Increased pressure |
Systemic hypertension |
Supravalvular aortic stenosis |
Pathophysiology
The presentation and findings in patients with AR depend on its severity and rapidity of onset. The hemodynamic effects of acute severe AR are entirely different from the chronic type, and the two will be discussed separately.
In response to the left ventricular volume overload associated with AR, progressive left ventricular dilation occurs. This results in a higher wall stress, which stimulates ventricular hypertrophy and which, in turn, tends to normalize wall stress. Patients with severe AR may have the largest end-diastolic volumes produced by any other heart disease and yet their end-diastolic pressures are not uniformly elevated. In keeping with the Frank-Starling mechanism, the stroke volume is also increased. Thus, despite the presence of regurgitation, a normal effective forward cardiac output can be maintained. This state persists for several years. Gradually, left ventricular diastolic properties and contractile function start to decline. The adaptive dilation and hypertrophy can no longer match the loading conditions. The left ventricular end-diastolic pressure begins to rise and the ejection fraction drops with a decline in effective forward output and development of heart failure.
In contrast to chronic AR, when sudden severe regurgitation occurs, the left ventricle has no time to adapt. The acute ventricular volume overload therefore results in a small increase in end-diastolic volume and severe elevation of end-diastolic pressure, which is transmitted to the left atrium and pulmonary veins, culminating in acute pulmonary edema. Because the ventricular end-diastolic volume is normal, the total stroke volume is not increased and the effective forward cardiac output drops. To compensate for the low output state, sympathetic stimulation occurs, which produces tachycardia and peripheral vasoconstriction, the latter further worsening AR.
Clinical Findings
Patients with chronic AR remain asymptomatic for a long time. Palpitations are common and may be due to either awareness of forceful left ventricular contractions or occurrence of premature atrial or ventricular beats. Angina may occur either from concomitant coronary disease or from a combination of low diastolic pressure and increased oxygen demand from ventricular hypertrophy. When left ventricular dysfunction supervenes, patients initially experience exertional dyspnea and fatigue. At a later stage, resting heart failure symptoms occur with orthopnea and paroxysmal nocturnal dyspnea.
On physical examination, visible cardiac pulsations are common. The area of the apical impulse is increased on palpation and is displaced caudally and laterally. The first heart sound is usually normal. The aortic component of the second heart sound may be decreased in conditions where cusp excursion is reduced, such as with valve calcification. An S4 is often present due to underlying hypertrophy, and an S3 is audible when ventricular failure occurs. On auscultation, the characteristic sound of AR is a soft, high-pitched diastolic decrescendo murmur best heard in the third intercostal space along the left sternal border at end expiration, with the patient sitting and leaning forward. In the presence of aortic root disease, the murmur may be best heard to the right of the sternum. A systolic ejection murmur may be present at the aortic area due to the high flow state. Occasionally, a diastolic rumble may be heard at the apex, referred to as the Austin Flint murmur. The mechanism underlying this murmur remains unclear. A number of different causes have been proposed, the most recent being the aortic jet encountering the mitral inflow resulting in turbulence.
The systolic arterial pressure is increased due to a large stroke volume, whereas the diastolic pressure is decreased due to runoff from the aorta into both the ventricle and peripheral arteries. This is the underlying reason for a wide pulse pressure and for a variety of associated peripheral signs in chronic significant AR (Table 18–2). However, it must be remembered that these signs are not specific for AR and may occur in any high flow state such as occurs in anemia, thyrotoxicosis, and arteriovenous malformations. With the development of heart failure, the pulse pressure narrows and the peripheral signs of AR are attenuated.
Name of Sign | Description |
|---|---|
Corrigan pulse | Rapid and forceful distention of arterial pulse with quick collapse |
De-Musset sign | To and fro head bobbing |
Müller sign | Visible pulsation of uvula |
Quincke sign | Capillary pulsations seen on light compression of nail bed |
Traube sign | Systolic and diastolic sounds (pistol shots) over the femoral artery |
Duroziez sign | Bruits heard over femoral artery on light compression by stethoscope |
Hill sign | Popliteal cuff pressure exceeding brachial pressure by 60 mm Hg or greater |
In contrast to chronic AR, most patients with acute severe AR are symptomatic. Initial presentation may vary depending on the underlying cause, which most commonly is aortic dissection, infective endocarditis, or trauma. In the presence of associated acute AR, clinical manifestations of severe dyspnea, orthopnea, and weakness often develop. The onset of symptoms is sudden, with rapid progression to hemodynamic collapse if left untreated.
In acute AR, the left ventricle has had no time to adapt to the volume overload state. The peripheral signs associated with chronic AR are therefore absent. Pulse pressure is usually normal, and hypotension may be present in severe cases. Bilateral rales are usually present on examination of the lungs and reflect underlying pulmonary edema. On precordial palpation, the apical impulse is not shifted. The first heart sound may be soft or absent due to the premature closure of the mitral valve. An S3 is often present, but an S4 is usually absent because there is little or no atrial contribution to ventricular filling due to high left ventricular end-diastolic pressure. The typical diastolic murmur of AR is shortened in duration, often difficult to hear, and easily missed.
Laboratory findings depend on the underlying cause of AR. Elevated white blood cell count and erythrocyte sedimentation rate are seen in inflammatory conditions, such as infection and aortitis. Abnormal antinuclear antigen and rheumatoid factor titers may be seen in patients with rheumatologic disorders. When syphilis is suspected, serologic tests may be indicated. Plasma brain natriuretic peptide (BNP) levels are associated with prognosis in patients with AR and may contribute to the decision for surgery.
No specific electrocardiographic abnormalities are characteristic of AR. Signs of left atrial enlargement, left ventricular hypertrophy, and a “strain pattern” (ST depression with T-wave inversion in lateral leads) are often seen in chronic significant AR. Arrhythmias, including ventricular ectopy and ventricular tachycardia, may occur in advanced cases with left ventricular dysfunction. In acute AR, sinus tachycardia may be the only abnormality. In cases of infective endocarditis, inflammation or abscess formation may spread to the atrioventricular node, resulting in prolongation of the PR interval or development of atrioventricular block.
Chest radiographic findings are not specific for AR and reflect an estimate of cardiac size and pulmonary vascular changes. In chronic significant AR, an increase in the size of the left heart chambers and the aorta is seen. In acute AR, the cardiac size is normal; the lung fields show increased markings due to pulmonary edema. When AR is due to aortic dissection, the chest film may show an enlarged ascending aorta. If calcification of the aortic knob is present, a helpful sign of dissection is increased separation between the outer margin of the aorta and the calcific density.
Echocardiography is the method of choice for evaluating patients with AR. Two-dimensional echocardiography in combination with various Doppler modalities and, in selected cases, transesophageal imaging has provided a noninvasive means for not only diagnosing AR with a high sensitivity and specificity but also for assessing its etiology and severity. Furthermore, important information can be obtained on the hemodynamic impact of the regurgitant lesion, prognosis, and effectiveness of therapy.
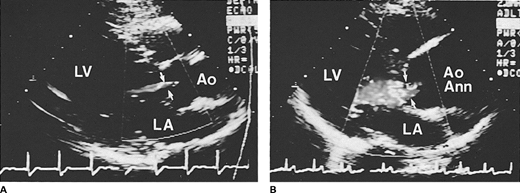
Doppler techniques are extremely sensitive and specific in the detection of AR, manifested as a diastolic flow abnormality arising from the aortic valve, directed toward the left ventricle. Even trivial regurgitation can be detected, which commonly is not audible on physical examination. Although most cases of moderate-to-severe chronic AR have typical findings on physical examination, moderate lesions may occasionally be missed on examination because of the subtlety of auscultatory findings. Doppler echocardiography is also extremely valuable in patients with acute AR when the typical clinical findings of chronic AR are absent and the brief murmur can often be missed. Color Doppler echocardiography has proven to be extremely helpful in the evaluation of AR (Figure 18–1). It provides a spatial orientation of the regurgitant jet arising from the aortic root. A completely negative color Doppler examination in multiple planes virtually excludes the presence of AR. Echocardiographic imaging with M-mode and two-dimensional examinations cannot detect the presence of AR but can provide indirect clues to its presence. These include diastolic fluttering of the anterior mitral leaflet or septum depending on the impingement of the regurgitant flow on these structures. These signs, although specific, are not sensitive for the detection of AR and do not relate to the severity of regurgitation.
Figure 18-1
Color Doppler echocardiographic frames in diastole from the parasternal long axis view in (A
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree