Fig. 21.1
Large abdominal aortic aneurysm. Notice how the majority of this aneurysm is filled with laminated thrombus. The flow lumen is only mildly dilated, and this aneurysm could be missed on angiography alone
Pathophysiology
Although the precise cause of aneurysmal degeneration of the aortic wall remains uncertain, atherosclerosis, cellular inflammation, and metalloproteinases have all been implicated [10]. Men are clearly at a higher risk for AAA, with a ratio of approximately 4:1 compared to women [11]. Other risk factors include age over 60, history of smoking, hypertension, emphysema, history of hernias, and family history. Family history is a particularly strong marker for patients who have a female first-degree relative with an AAA [12].
Developing an appropriate treatment plan for aneurysms requires a clear understanding of the difference between a true aneurysm and a pseudoaneurysm. Technically speaking, the number of vessel wall layers differentiates a true aneurysm from a pseudoaneurysm. Although this definition is precise and useful for pathologists, it cannot be applied clinically nor does it help in treatment planning. A more practical approach defines a true aneurysm as an abnormal expansion of the vessel due to defective structural proteins within the arterial wall. In contrast, a pseudoaneurysm occurs when a focal, transmural defect in the vessel wall allows extravasation of blood out of the artery and into the surrounding soft tissue where it is variably contained. Blood circulates into this “pulsatile hematoma” and back into the arterial lumen creating a characteristic “to-and-fro” flow pattern that is recognizable on color-flow ultrasound exam. Since pseudoaneurysms more accurately represent a contained rupture of the artery, they usually warrant swift intervention. The rate of expansion of pseudoaneurysms may be more rapid and less predictable than true aneurysms making treatment justified at almost any size [13]. In the periphery pseudoaneurysms tend to result from a traumatic injury or iatrogenic puncture. In the abdominal aorta, pseudoaneurysms often resemble a mushroom and stalk in appearance and are sometimes described as “saccular” aneurysms. Aortic pseudoaneurysms are believed to result from a focal rupture of a penetrating atherosclerotic plaque.
Most AAAs develop in the infrarenal aorta leaving a short segment of normal aorta below the renal arteries. This normal aortic segment or “aortic neck” allows for either open or endovascular aneurysm repair without compromising flow to the renal arteries. Juxtarenal aneurysms abut the renal arteries and lack a suitable infrarenal neck. Although surgery to repair juxtarenal aneurysms does not usually involve renal artery bypass or reconstruction, suprarenal clamping is required which increases the risk of postoperative renal dysfunction. Suprarenal aortic aneurysms require both suprarenal clamping and renal revascularization to achieve aneurysm repair and maintain renal blood flow. Repairing thoracoabdominal aortic aneurysms requires exposure in both the chest and abdomen and carries the highest risk of perioperative morbidity and mortality.
Perioperative Evaluation and Indications for Treatment
The risk of AAA rupture increases with the size of the aneurysm. Aneurysms greater than 6 cm in diameter have a rupture risk of approximately 10 % per year and generally warrant repair unless a patient has prohibitive comorbidities or a short life expectancy due to other clinical conditions [14, 15]. The optimal management of smaller aneurysms 4–5.5 cm in diameter has not been completely settled. Historically, patients with AAA greater than 5 cm were recommended for repair; however, several large prospective randomized trials suggest that patients with AAA less than 5.5 cm have a low risk of rupture and can be safely followed with surveillance imaging [16, 17]. Sound clinical judgment must be employed in each case as even small diameter aneurysms can rupture. For patients with a 5 cm AAA who have minimal cardiovascular risks and no evidence of malignancy, the aneurysm represents the single greatest threat to their life and continued well-being. Women and patients with COPD have a higher risk of rupture on a diameter-for-diameter basis and may warrant repair at a smaller AAA size. Likewise, patients with an AAA and symptoms of abdominal or back pain with no other obvious source are thought to have an increased risk of rupture and may require early repair. The morphology of a saccular aneurysm may predispose it to rupture and therefore warrant preemptive repair at a smaller diameter [18].
Aneurysms grow at an average rate of approximately 10 % per year. Aneurysms with a significantly more rapid growth rate should be considered for repair even when they are still small in diameter [19]. Patients who are being followed with surveillance imaging should stop smoking and aggressively control their blood pressure since smoking and hypertension are the only modifiable risk factors associated with aneurysm expansion and rupture [20].
Patients with AAA should be evaluated for aneurysms in other anatomic locations. Popliteal artery aneurysms occur in more than 5 % of patients with AAA, particularly in elderly men [21]. If the popliteal pulse is generous on physical exam, an ultrasound can easily measure the popliteal artery diameter to determine if there is an aneurysm. Recent studies suggest that as many as 25 % of patients with AAA may have a concurrent thoracic aortic aneurysm [22]. Patients with a known AAA being evaluated with CT scan should have a comprehensive scan including the thoracic aorta, particularly women and elderly patients with AAA.
A high-quality CT angiogram should be performed in all patients with AAA who are being considered for treatment, unless they have a major contraindication. In patients with renal dysfunction, a non-contrast CT of the abdomen and pelvis with thin axial cuts (less than 1 mm) can provide valuable anatomic information. CT scanning is essential to determine whether an endovascular repair will be technically feasible, but CT scans also help plan open surgical repair. CT scan demonstrates the proximal extent of the aneurysm to insure that there is no extension above the renal arteries or a coexisting aneurysm involving the distal thoracic aorta or the visceral segment of the abdominal aorta. The celiac trunk and superior mesenteric artery (SMA) should be evaluated to exclude significant mesenteric disease, since the inferior mesenteric artery (IMA) is typically sacrificed as part of the open surgical repair. The presence of severe stenosis or occlusion of the SMA with an enlarged IMA (“meandering mesenteric”) might necessitate reimplantation of the IMA into the body of the aortic graft at the time of open repair.
The CT scan can also detect other important abdominal pathology, like an undiagnosed malignancy. The major venous structures should be examined to identify anatomic variants such as a duplicated or left-sided inferior vena cava (IVC), retroaortic renal vein, or a renal venous “ring.” These venous anomalies can be the source of troublesome intraoperative bleeding during dissection and proximal aortic control if they are undetected preoperatively.
A CT scan which shows thick, contrast-enhancing soft tissue encompassing the anterior 270° of the aorta may indicate an inflammatory aneurysm which will be discussed in more detail below. Tissue resembling renal parenchyma on the anterior surface of the aorta may represent a horseshoe kidney. Additionally, the aorta should be evaluated for the presence of calcification, particularly at the aortic bifurcation and in the iliac vessels. The iliac vessels themselves should be inspected for the presence of atherosclerotic occlusive disease which could impede passage of an endovascular stent graft or prohibit aortic reconstruction with a prosthetic tube graft. Approximately 10–20 % of patients with an infrarenal AAA have aneurysms involving the common or internal iliac arteries.
Measuring the ankle-brachial index (ABI) provides objective, preoperative documentation of lower extremity arterial perfusion. If the ABI is significantly abnormal, preoperative angiography should be considered to clarify the precise anatomic location and severity of the occlusive lesions. The presence of severe aortoiliac occlusive disease often modifies the plan for surgical repair. Routine noninvasive evaluation of the carotid arteries should also be considered in patients with associated cardiovascular risk factors [23]. Patients with undiagnosed severe internal carotid artery stenosis pose a higher perioperative stroke risk for major open abdominal surgery [24].
In addition to the standard cardiac and non-cardiac risks inherent in any major intracavitary procedure, patients with AAA should be aware of the complications specific to aneurysm repair surgery. Potential complications of AAA surgery include distal embolization or thrombosis in the lower extremities, bowel ischemia, and acute kidney injury. In men, sexual dysfunction in the form of either erectile dysfunction or retrograde ejaculation can occur and appears to be related to the amount of dissection around the autonomic nerves which travel near the infrarenal aorta and left common iliac artery.
Open Surgical Repair
Open AAA repair may be performed through a longitudinal midline or mid-abdominal transverse incision. Retroperitoneal exposure using a left flank incision has also been used and may be favored in cases of hostile abdomen (previous complex abdominal surgery, stoma, etc.) and inflammatory aneurysm (discussed below) and selected patients with severe COPD that are not candidates for an endovascular approach. Advantages of transabdominal exposure are direct evaluation of all intra-abdominal viscera both before and after repair and more predictable access to the right iliac arteries if required. Table 21.1 compares the major advantages and drawbacks of transabdominal and retroperitoneal exposure of the abdominal aorta.
Table 21.1
Advantages and drawbacks of transperitoneal vs. retroperitoneal exposure for open repair of abdominal aortic aneurysm
Transabdominal | Retroperitoneal | |
|---|---|---|
Advantages | Rapid exposure | Avoid hostile abdomen |
Widest access | Better juxtarenal control | |
Intra-abdominal evaluation | Shorter ICU stay | |
Drawbacks | Longer ileus | Poor access to right side |
Greater fluid loss | Flank bulge and incisional pain | |
More pulmonary dysfunction | Longer open/close time | |
Higher cost | No intra-abdominal evaluation |
To achieve transperitoneal exposure of the infrarenal aorta, the transverse colon is reflected cephalad and the small bowel is retracted to the patient’s right. The retroperitoneal tissue is incised over the aneurysm, and the duodenum is mobilized off the aorta and to the patient’s right. The inferior mesenteric vein (IMV) runs parallel to the aorta on the left. Dissection continues superiorly on the anterior surface of the aorta to the level of the left renal vein; if the IMV compromises exposure at that level, it can be divided. In most cases proximal control of normal caliber aorta is possible at the level of the left renal vein. If more proximal aortic exposure is necessary, the left renal vein can be divided toward its confluence with the inferior vena cava, taking care to preserve the adrenal and gonadal venous tributaries.
Distal vascular control is then achieved by isolating the common iliac arteries. If the iliac arteries are not involved with aneurysmal or occlusive disease, a tube graft repair of the AAA can be performed. Common iliac artery aneurysms require repair with an aortobi-iliac bypass which is usually anastomosed to the distal common iliac artery or iliac bifurcation. The presence of significant iliac artery occlusive disease usually mandates an aortobifemoral bypass to avoid postoperative lower limb ischemia. This type of vascular reconstruction requires bilateral groin incision for exposure of the femoral arteries.
After suitable proximal and distal control of the vessels has been obtained, systemic heparin is administered prior to clamping. The aneurysm sac is then opened longitudinally and all thrombotic debris removed. Back bleeding from patent lumbar arteries and the IMA can be controlled by oversewing the vessel orifices from inside the aneurysm sac. If the IMA is large (particularly if there is known disease of the SMA) or has sluggish back bleeding, reimplantation of the IMA onto the body of the aortic graft using a Carrell patch should be considered to avoid postoperative colon ischemia.
The prosthetic graft used most often for AAA repair is a woven polyester textile (Dacron) graft, but polytetrafluoroethylene (PTFE) grafts are also available. Grafts are sewn in place with 3-0 or 4-0 nonabsorbable monofilament sutures (usually polypropylene). When graft repair has been completed, the wall of the aneurysm is closed over the graft, and the remainder of the retroperitoneal tissue reapproximated over the aneurysm sac. Prior to wound closure, the viability of the abdominal viscera, particularly the left colon, should be inspected.
Inflammatory Abdominal Aortic Aneurysms
The etiology of inflammatory aneurysms remains uncertain. Patients tend to be younger and more frequently present with abdominal symptoms [25]. The liberal use of CT scanning in modern practice increases the chances of detecting an inflammatory AAA preoperatively. A CT showing a contrast-enhancing, thickened rind of inflammatory tissue around the aneurysm suggests the possibility of an inflammatory AAA. Inflammatory AAA may be associated with retroperitoneal fibrosis with involvement of the ureters and hydronephrosis [26]. Suspicion of an inflammatory AAA based on preoperative imaging studies warrants an endovascular repair if it is anatomically feasible. If open AAA repair is required, a retroperitoneal approach should be considered to avoid dissection in the inflammatory tissue surrounding the anterior aorta.
The typical appearance of an inflammatory aneurysm encountered during transperitoneal exploration is a pearly, milky-white, glistening surface along the entire anterior wall of the aneurysm. This is almost always associated with firm adherence of the duodenum to the aneurysm wall, and no attempt should be made to free the duodenum from this surface. The inflammatory process rarely extends above the body of the aneurysm, and it is often possible to gain proximal infrarenal aortic control. Alternatively, temporary suprarenal or supraceliac aortic control can be used. The inflammatory process frequently involves the common iliac arteries, so direct distal control of those vessels may not be possible. In this situation, control of the iliac arteries is achieved with intraluminal occlusion balloons placed after opening the aneurysm sac with a proximal clamp in place. Graft repair then continues from within the AAA lumen. Following open or endovascular repair of an inflammatory AAA, the retroperitoneal fibrosis may spontaneously resolve [27]. Most aspects of inflammatory AAA including its cause and resolution remain poorly understood.
A mycotic aneurysm of the abdominal aorta is not a true aneurysm but an infected pseudoaneurysm caused by suppurative autolysis of the aortic wall. These rare aneurysms represent a “contained” rupture and should be treated aggressively at any size. Mycotic aneurysms can often be recognized by their atypical morphology on CT scan, appearing as a focal, saccular outpouching along the course of an otherwise disease-free aorta [28]. Suspicion for a mycotic aneurysm should increase in young patients without other risk factors for aneurysm. Patients with mycotic aneurysms may report a prodrome of systemic symptoms (e.g., fever, malaise, gastrointestinal symptoms) prior to presentation. The most common bacteria causing mycotic aortic aneurysms are Salmonella species and Staphylococcus aureus. If gross purulence is encountered at the time of surgery, it is probably safest to oversew the aorta proximally and distally and debride as much involved aortic wall as possible. Limb revascularization is then provided by constructing an extra-anatomic axillobifemoral bypass [29]. When gross purulence is not present, in situ aortic reconstruction may be considered. This can be performed with an antibiotic-soaked graft (most often rifampin), a cryopreserved aortic homograft, or an autologous reconstruction using deep femoral veins harvested from the legs [30, 31]. In all cases of mycotic aortic aneurysm, long-term antibiotic treatment should continue postoperatively [28].
Primary Aortocaval Fistula
Rarely, brisk venous bleeding from the aortic wall is encountered after opening the aneurysm sac. This clinical scenario represents an aortocaval fistula caused by chronic erosion of the AAA into the adjacent IVC [32]. No attempt should be made to dissect the aortic wall from the IVC as this will likely cause more bleeding. Instead, direct pressure proximal and distal to the fistula can provide temporary hemostasis allowing the defect to be sutured closed from within the aneurysm sac. An aortocaval fistula is almost always associated with a hyperdynamic cardiopulmonary status, so the anesthesiologist should be warned that preload will significantly decrease when the fistula is closed.
Ruptured Abdominal Aortic Aneurysms
Left untreated, a ruptured AAA is universally fatal. Emergent surgical or endovascular repair offers the only hope of survival. Most modern emergency facilities in the United States have rapid CT scanners capable of imaging the abdominal aorta within minutes. Even without intravenous contrast, a CT scan can diagnose a ruptured AAA and determine the options for repair (Fig. 21.2). In centers with appropriate personnel and equipment, endovascular repair of ruptured AAA has reduced mortality and morbidity [33]. Preparations for an open surgical repair should address several key logistical and technical issues. Blood and blood products (fresh-frozen plasma, platelets) should be readily available. Since patients with ruptured AAAs usually present with hypotension, resuscitation efforts will have already begun in the emergency department. These efforts should be closely monitored with the goal having a responsive patient with a systolic pressure of 70–90 mmHg. Maintaining “permissive hypotension” until bleeding can be definitively controlled is more desirable than aggressive resuscitation which can lead hypertension, increased bleeding, and rapid deterioration of the patient [34].
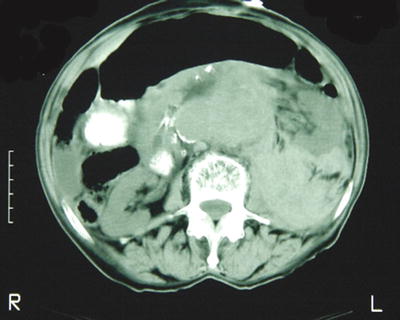

Fig. 21.2
Classic image of a ruptured abdominal aortic aneurysm, which in this case can be seen even without the use of intravascular contrast. Note the lack of symmetry and obliteration of tissue planes in the retroperitoneum on the left. Although contrast is not necessary to diagnose an AAA rupture, it is helpful in determining the candidacy for and planning of endovascular repair
In the operating room, sterile skin preparation and draping should extend from the upper chest to the knees in cases where exposure and control of the thoracic aorta are required. Ideally, the patient should be prepped and draped prior to induction as muscle relaxation can release the retroperitoneal tamponade causing sudden hypotension or cardiovascular collapse. Proximal control of the aorta is always the initial objective of surgery. In most cases the large retroperitoneal hematoma makes it impossible to directly control the infrarenal aorta without causing a significant venous injury, usually to the left renal vein. Initial proximal control should be achieved at the supraceliac aorta which can be exposed by incising the triangular ligament to retract the left lobe of the liver, dividing the gastrohepatic ligament, and dividing or separating the fibers of the diaphragmatic crus directly overlying the aorta. It is helpful to have a nasogastric or orogastric tube placed for easy identification of the esophagus while dividing the fibers of the crus of the diaphragm.
With a supraceliac aortic clamp in place, a more controlled dissection can be carried down through the hematoma to identify and isolate the normal aortic segment proximal to the aneurysm. The aortic clamp can then be moved to this “aortic neck” to maintain hemostatic control while restoring perfusion to the viscera and kidneys. When infrarenal control has been established, full resuscitation can ensue. The aneurysm sac should be widely opened, and distal control of the iliac arteries can be initially achieved using intraluminal balloon occlusion catheters. If the retroperitoneal hematoma does not extend into the pelvis, direct dissection and control of the iliacs may be possible. Aortic reconstruction with a prosthetic tube graft is usually preferred if it is anatomically possible to decrease the duration of surgery.
Postoperatively patients require close monitoring in the intensive care unit, as the risks of abdominal compartment syndrome and colon ischemia are considerably higher than for elective AAA repair. If there is any concern at the completion of the procedure in terms of tightness of closure or elevation of peak airway pressures, consideration should be given to leaving the abdomen open. These cases are managed in a similar fashion to “damage control” surgery with temporary vacuum-assisted abdominal closure devices and delayed fascial closure when edema resolves [35]. If the abdomen is initially closed but the patient manifests symptoms of abdominal compartment syndrome postoperatively, bladder pressures can approximate intra-abdominal pressure [36]. Persistently high bladder pressures in a patient with elevated airway pressures, abdominal distention, difficulty with ventilation, oliguria, or unexplained hypotension should warrant an immediate return to the operating room. Reopening the abdominal cavity to relieve the abdominal compartment syndrome can be lifesaving.
Conclusion
Abdominal aortic aneurysms remain a serious clinical problem in our aging population. Ultrasound screening of appropriately selected people at risk can significantly reduce AAA-related mortality by detecting aneurysms early in their course. Endovascular therapy (discussed elsewhere) has emerged as a minimally invasive method of AAA repair which significantly reduces morbidity and mortality compared to open repair. Although endovascular surgery now accounts for 60–70 % of all AAA repairs, many patients will still require open surgery because of unsuitable anatomy or clinical circumstances. Surgeons managing these cases must have a working knowledge of the anatomy, techniques for aortic control, and vascular reconstructive alternatives to successfully repair an abdominal aortic aneurysm.
Aortic Dissection
Introduction
Acute aortic dissection is a relatively common condition affecting the thoracic or abdominal aorta that carries a high mortality rate if left untreated. A dissection involves an abnormal blood flow which separates the layers of the vessel wall. A focal intimal tear or injury usually triggers a dissection by allowing blood flow to gain entry and track between the muscular layers of the aortic wall. The extent and clinical severity of a dissection depend on multiple factors including location and size of the intimal injury, systemic blood pressure, and preexisting vascular disease.
The term “dissecting aneurysm” is often the source of considerable confusion and is thus worth addressing. Although a dissection and an aneurysm can occasionally occur in the same patient, it is important to note that they are two completely different clinical entities. The use of this misnomer is probably related to the association between chronic dissection and an increased incidence of aneurysm formation over time. This section will review the classification, epidemiology, physiology, clinical presentation, diagnosis, and treatment of acute aortic dissection, with a focus on care of these patients from the general surgeon’s perspective.
Classification
The anatomic location of an acute aortic dissection usually determines its treatment pathway. The Stanford model has emerged as the simplest and most widely used classification system for aortic dissections (Fig. 21.1). A Stanford type A dissection is any dissection involving the ascending aorta, whereas Stanford type B encompasses all dissections which do not involve the ascending aorta. The distinction between Stanford type A and B dissections is of critical importance as the prognosis and management algorithms differ. The DeBakey model offers a slightly more detailed classification system (Fig. 21.3).
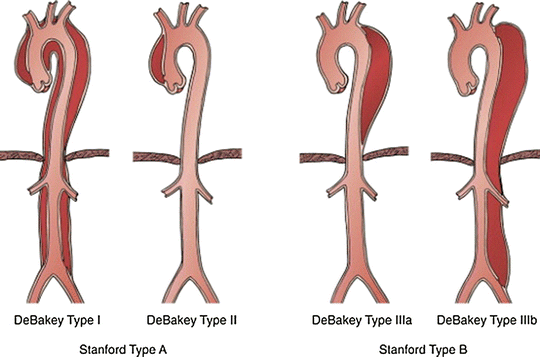

Fig. 21.3
Classification system for thoracic aortic dissection
Epidemiology
The incidence of acute aortic dissection in the United States ranges from 2.9 to 3.5 per 100,000 person years. Risk factors for developing an aortic dissection include advanced age, hypertension, and smoking. Men are more frequently affected, with a male-to-female ratio of 4:1. Type A dissections occur nearly twice as often as type B dissections (62.5 % versus 37.5 %). Other risk factors for dissection include aortic wall structural abnormalities, the presence of a bicuspid aortic valve, and cocaine abuse. Congenital conditions such as coarctation of the aorta, annuloaortic ectasia, aortic arch hypoplasia, and hereditary collagen vascular conditions (Marfan syndrome and Ehlers-Danlos syndrome) have also been implicated. Marfan syndrome accounts for 50 % of cases of acute aortic dissection in patients younger than 40 years. Pregnancy is also a well-known risk factor for aortic dissection and may be related to the severe hypertension that occurs in women with preeclampsia.
Pathophysiology
A dissection can occur in any artery and is almost always related to an intimal injury or tear which acts as an entry point to the space between the layers of the arterial wall. Systemic arterial pressure drives blood flow between the arterial muscle layers causing the dissection to propagate. The extent and severity of the dissection depend on the size of the intimal tear, systemic arterial pressure, proximity to branch points, and the development of reentry pathways. The dissection plane is referred to as the “false lumen” to differentiate it from the normal pathway for blood flow or “true lumen.” In type B dissections, the intimal tear most commonly occurs at the level of the left subclavian artery because it is exposed to the greatest pressure fluctuations and shear stress. The dissection plane usually extends distally along the left posterolateral aspect of the aorta with the celiac, superior mesenteric, and right renal arteries being perfused by the true lumen, while the left renal artery arises from the false lumen. Although this is the most common flow pattern, any combination of visceral artery involvement is possible.
In type A dissection, the dissection flap starts in the ascending aorta and may propagate retrograde. Mortality in type A dissection typically involves one of four possible mechanisms: (1) The dissection flap may propagate into the coronary arteries, causing flow compromise and acute myocardial infarction. (2) The dissection flap may result in expansion of the pericardial sac causing cardiac tamponade. (3) The dissection may disrupt the valve apparatus causing severe aortic insufficiency. (4) The dissection may extend into the carotid arteries resulting in acute stroke.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


