Aortic Dissection—Types A and B
Katherine B. Harrington
D. Craig Miller
Dissection of the aorta is characterized by separation of the aortic wall in the outer third of the media thereby creating a false lumen in the aortic wall that parallels the true lumen. A PIT* almost always initiates the dissection and allows blood flow into the false lumen; one or usually multiple distal spontaneous fenestrations in the dissection flap can allow reentry into the true lumen, which decompresses the false lumen.
IMH and PAUs are pathologic variants of classical aortic dissection. In contrast to a dissection, neither IMH nor PAU has blood flow in the false lumen. Thoracic aortic IMH is treated using the same rationale that we use for a genuine dissection.
The most common presenting symptom is abrupt onset, sharp, severe, chest or back pain. Initial chest x-ray may show mediastinal widening or abnormal aortic contour in 56% of patients. Pulse deficits are present in approximately 10% of patients. Absence of abrupt pain, pulse changes, and mediastinal widening makes the diagnosis of acute dissection rare (4% of patients). Patients tend to be hypertensive when they present; if hypotensive, rupture and/or tamponade may already have occurred.
Classification
Historically, dissections have been considered “acute” in the first 14 days after onset of symptoms, and “chronic” thereafter as patients typically stabilize after this time and are managed more conservatively. More recently investigators have expanded these definitions to include “subacute” (out to 90 days) and even “hyperacute,” but further research is needed to learn if this subcategorization has any meaningful clinical impact.
There are several anatomic classification schemes for describing aortic dissections, but the most widely used is the Stanford nomenclature, which considers all dissections involving the ascending aorta proximal to the innominate artery to be Type A; dissections which involve only the descending aorta and arch are Type B dissections. The DeBakey classification classifies dissections into three groups: Type I and II (confined solely to the ascending aorta) are those where the ascending aorta is involved. DeBakey
Type III dissections involve the descending thoracic aorta and are subdivided into Type IIIa (descending thoracic aorta only) and Type IIIb (extending into abdominal aorta). The incidence of aortic dissection is approximately 3 in 100,000, with roughly two-thirds of these being Type A dissections.
Type III dissections involve the descending thoracic aorta and are subdivided into Type IIIa (descending thoracic aorta only) and Type IIIb (extending into abdominal aorta). The incidence of aortic dissection is approximately 3 in 100,000, with roughly two-thirds of these being Type A dissections.
Indications for Different Treatment Options
Emergent surgical aortic repair is recommended for patients with acute Type A dissection. The optimal treatment strategy for patients with acute Type B dissection continues to evolve: The therapeutic mainstay is conservative medical management. Results of medical treatment are equal if not better than after urgent surgical repair, but the patient groups are not comparable. Most institutions favor a “complication-specific” approach, reserving surgical or thoracic aortic stent grafting (TEVAR and/or branch true lumen stenting) for those with “complicated” dissections, which after the FDA approval of TEVAR for dissection is now rigorously defined as malperfusion, rupture, or impending rupture. The older “soft” indications (persistent pain, refractory hypertension) no longer make an acute Type B dissection complicated.
TEVAR in uncomplicated patients with acute/subacute Type B dissections is being explored and has shown promise in reducing late aortic complications and accelerating aortic remodeling. It can be considered especially if there are risk factors for late aneurysm development including proximal location of PIT, a large PIT (>10 mm), and aortic size (true and false lumen) >45 mm.
In patients with complicated acute Type B dissections, the surgeon must decide between an open versus an endovascular approach. TEVAR is the preferred first-line therapy today; however, younger patients, good surgical candidates, patients with the MFS and other connective tissue diseases, and patients with unfavorable TEVAR anatomy should be considered for open surgical descending thoracic aortic repair.
Surgical aortic repair for patients with chronic Type B dissections is indicated for both symptomatic patients (pain, distal ischemia) and asymptomatic patients (rapidly expanding or >6 cm aortic aneurysmal degeneration). While practiced extensively today, there is a paucity of information supporting TEVAR for patients with chronic Type B dissections, and no randomized controlled data exist. Due to the multiple fenestrations in the dissection flap, there is no guarantee the false lumen will thrombose and become depressurized, thereby providing some protection from aortic rupture. Nonetheless, TEVAR can be performed in patients with symptomatic chronic Type B dissections and those where the aortic diameter exceeds 5.5 cm.
There are few contraindications to emergent surgical repair of an acute Type A aortic dissection because death is almost inevitable if untreated; however, those with a severe stroke or deep coma (especially 6 hours after initial presentation) or very frail, elderly patients with other medical problems should not be offered operation due to futility. TEVAR for patients with Type B dissections as a definitive treatment should be avoided in patients with MFS or other connective tissue disorders (a contraindication in all the TEVAR trials) in favor of open surgical aortic repair. However, if a patient is in extremis due to rupture or severe malperfusion, then it is reasonable to treat them with emergency TEVAR in an attempt to resuscitate the patient as a temporizing measure.
Initial diagnostic procedure options include CTA scanning, TEE, and in chronic cases magnetic resonance angiography. Study interpretation should include dissection classification, extent, PIT location, and presence/absence organ malperfusion. Further information needed for possible TEVAR insertion includes the dimensions of descending
aorta, size of true and false lumen, arch branch vessel anatomy, potential landing zones, and femoral/iliac dimensions for access.
aorta, size of true and false lumen, arch branch vessel anatomy, potential landing zones, and femoral/iliac dimensions for access.
As soon as acute aortic dissection is suspected, emergency medical therapy should be initiated and continued while the diagnostic procedures are performed. Medical treatment includes reduction of mean, peak, and rate of rise arterial pressure (dP/dt) with both an intravenous (a) β-blocker (esmolol, labetalol) and (b) vasodilator (nitroprusside).
Acute Type A Dissection: Surgical Management
The goal of surgical correction is to excise the PIT, redirect flow into the true lumen, and remove the risk of coronary malperfusion, AR, and ascending rupture by replacing the ascending aorta and most of the arch. Aortic root and valve replacement may also be necessary.
Cannulation Strategy
Avoid femoral retrograde CPB cannulation whenever possible. Cannulating in the groin for retrograde perfusion may pressurize the false lumen and cause descending rupture and increase the risk of stroke. In chronic dissections, it may embolize thrombus and debris from the thoracoabdominal aorta into the cerebral circulation. If absolutely necessary in an emergency situation, cannulate the femoral artery on the side with a weak or absent pulse as this artery is more likely to perfuse the aortic true lumen.
The right axillary artery is our preferred location for arterial CPB inflow: it provides antegrade flow to the body, makes it very simple to institute SACP during circulatory arrest, and allows the security of arterial cannulation prior to opening the chest in cases of tamponade or contained rupture.
If the axillary artery is not useable, other antegrade perfusion options include cannulating the true lumen in the arch directly or TA LV cannulation across the aortic valve. Both require TEE guidance and the Seldinger technique assuring the distal tip of the cannula is in the aortic true lumen while cooling.
Standard venous cannulation with a two-stage venous cannula through the right atrial appendage is sufficient.
Myocardial Protection
Initial arrest should be retrograde cardioplegia. Do not inject pressurized antegrade cardioplegia into a dissected aorta. With the retrograde running, the aorta is opened.
Antegrade cardioplegia may then be given down each coronary ostium cautiously using atraumatic cannulas. This should be avoided if the dissection flap extends into the coronary artery itself.
Myocardial protection is achieved with intermittent retrograde cardioplegia, topical myocardial hypothermia, and systemic hypothermia.
If the patient presents with ST changes or other signs of severe myocardial ischemia, the saphenous vein can be exposed at the beginning of the operation in case coronary artery bypass grafting is needed.
Once the patient is on CPB, systemic cooling is initiated immediately. The head is packed in ice, and mannitol, furosemide, and dexamethasone are administered. The patient is cooled to a tympanic membrane temperature of 18º to 20ºC, especially if extensive arch work is planned. There are some who use moderate systemic hypothermia (25º to 28ºC) and claim acceptable neurologic outcomes when SACP is used if the circulatory arrest time is short, that is, for an open oblique distal anastomosis at the innominate artery level (which is a suboptimal procedure). We, on the other hand, prefer extended partial arch replacement from the level of the ligamentum on the distal lesser curvature to the innominate artery in order to remove as much traumatized aorta
as possible and any tears in the arch. It is often not clear prior to inspecting the inside of the arch what extent of arch replacement will be needed, so it is prudent always to cool to 18º to 20ºC if the dissection extends into the arch. If severe AR is not present, we prefer to wait until the pump is turned off and avoid an ascending aortic clamp; if this is not possible, then the clamp should be applied as proximally as possible.
as possible and any tears in the arch. It is often not clear prior to inspecting the inside of the arch what extent of arch replacement will be needed, so it is prudent always to cool to 18º to 20ºC if the dissection extends into the arch. If severe AR is not present, we prefer to wait until the pump is turned off and avoid an ascending aortic clamp; if this is not possible, then the clamp should be applied as proximally as possible.
While cooling, the ascending aorta, aortic root, and arch vessels are dissected out. Avoid electrocautery around the distal arch to avoid damage to the recurrent laryngeal nerve, which can be difficult to visualize in the hematoma. Vessel loops are placed around the innominate, left carotid, and left subclavian arteries. Once the patient is cold enough he/she is placed in a steep Trendelenburg position, the pump flow is reduced to 10 mL/kg/min, the ascending aorta is opened, the arch branches are clamped thereby commencing SACP, and the heart arrested with retrograde cardioplegia as described above. Cerebral NIRS is monitored bilaterally. The mid ascending aorta is opened longitudinally taking care to note the extent of the PIT, which is most commonly along the greater curve of the aorta immediately distal to the sinotubular junction. If minimal left carotid back flow is seen or the NIRS or tympanic membrane temperatures are asymmetric, a 13-Fr pediatric retrograde cardioplegia cannula is placed up the left carotid artery and Y’ed to the arterial CPB line for bilateral cerebral perfusion.
Aortic Arch Management
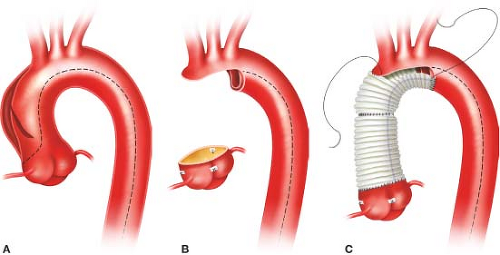
We always perform an open distal arch anastomosis, but admit there are no randomized controlled studies to support this practice. The aorta is trimmed to expose the entire arch. Major reentry tears or a PIT in the transverse arch dictate total arch replacement with a multibranched graft. More commonly the head vessels and greater curvature of the arch can be left as a peninsula using a single long sigmoid-shaped suture line (Fig. 11.1). A woven double-velour Dacron graft is beveled and rotated 180 degrees, such that the “toe” of the graft is aligned along the greater curvature of the arch at the innominate artery; this keeps the lesser curvature of the graft as short as possible which prevents excessive graft length and graft buckling or folding. The distal anastomosis is performed with a running 5-0 C1 or 4-0 BB Prolene suture to carefully construct a full-thickness end-to-end anastomosis incorporating all layers circumferentially. Completely obliterating the distal false lumen is usually impossible as it extends far distally down the descending aorta. We do not use Bioglue in the distal false lumen to obliterate it as the glue may embolize downstream and there is a chance of causing aortic necrosis, which can potentially result in an anastomotic pseudoaneurysm late postoperatively. We do not use strips of Teflon-felt to buttress this distal arch anastomosis, but some surgeons still do. After completion of the distal anastomosis, the arch is de-aired, the arch branches unclamped, and arch graft is reclamped. Full flow CPB is then reinstituted, and systemic rewarming commenced after 10 minutes.
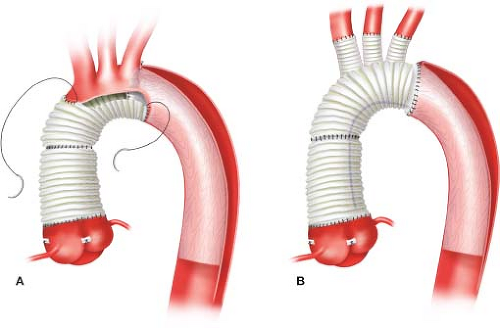
More aggressive approaches to treat the distal dissection are evolving. In addition to performing an extended partial arch replacement in patients with acute Type A dissections, Alberto Pochettino and Eric Roselli have championed concomitant antegrade implantation of a FET graft into the distal descending aortic true lumen using a stent graft (Gore c-TAG, W. L. Gore, Flagstaff, AZ) based on the notion that this would promote more thrombosis of the distal false lumen and potentially reduce the need for subsequent “downstream” aortic reinterventions (Fig. 11.2A




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree