Aortic Arch/Great Vessel Surgery
Joseph S. Coselli
Introduction
The aorta is divided into two major segments—the proximal aorta and the distal aorta. The proximal aorta includes the aortic root, ascending aorta, and transverse aortic arch. The transverse aortic arch (Fig. 12.1) is anatomically defined as the segment of aorta that includes the origins of the brachiocephalic vessels: The innominate artery, left common carotid artery (LCCA), and left subclavian artery (LSCA).
Aortic arch repairs are typically performed to treat aortic aneurysm, dissection, or both. Arch aneurysms tend to occur as extensions of adjacent disease involving the ascending and/or the descending thoracic aorta. Acute aortic arch dissection may arise antegrade from a tear in the ascending aorta (usually a few centimeters above the origins of the coronary arteries) or retrograde from a tear in the descending thoracic aorta (typically just distal to the LSCA); rarely, the originating tear is in the arch itself or in the arch vessels. Over time, chronic aortic dissection may facilitate dilatation of the aortic arch and necessitate repair.
All aneurysms are repaired to prevent fatal rupture. Although replacing the aorta can be life saving in patients with aortic disease, operative risk must be balanced against indications for repair. In asymptomatic patients with known aortic disease, imaging studies are used to carefully monitor the aorta until diameter-based thresholds of repair are met or until symptoms develop. Current guidelines make several scenario-based recommendations for the repair of aortic arch aneurysms, with the caveat that repair is generally dictated by adjacent disease. For isolated arch aneurysms in low-risk, asymptomatic patients, it is reasonable to replace the arch when the aortic diameter exceeds 5.5 cm or is growing more than 0.5 cm per year. Replacing the entire arch is also reasonable in cases of chronic dissection combined with aneurysm and in cases of acute aortic dissection with extensive arch destruction and leakage.
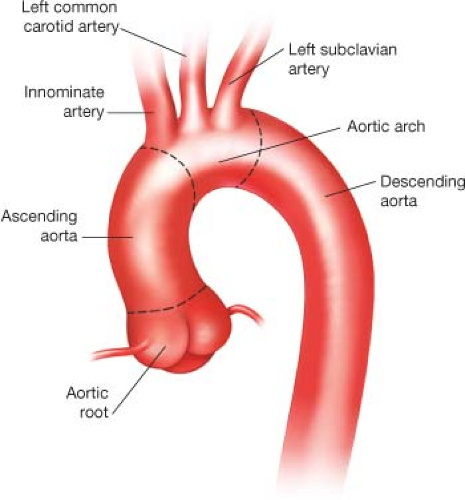 Figure 12.1 Illustration of normal aortic arch anatomy. The brachiocephalic vessels arise from the transverse aortic arch and serve as anatomic landmarks. |
Symptoms
When specific symptoms are present, they are usually related to aneurysmal expansion and compression of surrounding structures or to malperfusion related to aortic dissection. The onset of symptoms is usually considered an indication of impending rupture or significant malperfusion and should prompt urgent evaluation and repair. Patients may have dysphagia from esophageal compression, respiratory symptoms due to airway compression, or edema of the upper body from superior vena caval compression. Other problems that can arise from aneurysms involving the aortic arch and the adjacent segments include thromboembolic events such as stroke, and aortic valve regurgitation from associated root enlargement. Chest wall compression can cause chronic dull or aching retrosternal or mid-scapular pain. New severe pain usually indicates aneurysm rupture or acute dissection. Arch aneurysms can rupture into the pleural cavity (usually on the left), mediastinum, esophagus, or tracheobronchial tree. Common scenarios in which aortic dissection occurs include uncontrolled hypertension, connective tissue disorders (such as Marfan syndrome), and bicuspid aortic valve; aortic dissection may cause cerebral malperfusion and result in symptoms such as syncope and neurologic deficits.
Full aortic arch replacement in patients with acute aortic dissection is somewhat controversial. The traditional approach in such patients is to perform a partial arch resection and hemi-arch repair (see Chapter 10, which covers this approach). Contraindications to open aortic repair are patient-specific and individualized to the experience of each surgeon. In general, patients with limited life expectancy are not candidates for open surgical repair, although aortic repair in survivable forms of cancer may be warranted before its treatment. Although patients at prohibitive risk of open surgical repair are sometimes offered hybrid endovascular repair, such repair is not without substantial risk, and open aortic arch repair remains the gold standard for treatment. In addition, patients should be presented with information regarding the risk of developing life-altering complications such as stroke or renal failure resulting in dialysis dependence.
Except in those patients who require emergent repair, all patients should undergo a thorough preoperative evaluation emphasizing cardiac, pulmonary, and renal function, as well as a careful review of imaging studies. The diameter of the aorta throughout the diseased and nonaneurysmal portions is determined, and potential sites for cannulation are reviewed for calcification, dissection, and mural thrombus. Cannulation strategies may need to be adjusted in patients on the basis of preoperative findings, such as anomalies of branching arch vessels. The risk of stroke is significant during aortic arch repair, and because there is a well-known association between aneurysmal aortic disease and carotid artery stenosis, it is useful to perform a screening ultrasound of these arteries. Additional investigation may include pulmonary function testing, cardiac stress testing, nuclear imaging, and coronary catheterization. When significant coronary artery disease is identified in an elective surgical candidate, myocardial revascularization is recommended before aortic replacement.
Preoperative renal dysfunction is associated with early postoperative mortality and morbidity after aortic repair. While computed tomography (CT) imaging studies are quite useful in planning repair, the nephrotoxic contrast agents risk damaging renal function. In patients with borderline renal function, preventative measures are taken before imaging studies are conducted. A solution of 5% dextrose and Ringer’s lactate solution with 25 g/L mannitol and 1 amp/L sodium bicarbonate is typically administered intravenously before image acquisition. Acetylcysteine may be administered before and after such studies to further reduce the risk of contrast-induced nephropathy. After CT scanning or aortography, whenever possible, surgery is delayed for 24 hours or longer until renal function has returned to baseline.
Cerebral Protection
The arch is particularly challenging to repair because—unlike other aortic sections—its replacement usually necessitates interrupting both cerebral and distal aortic perfusion, leaving the brain, spinal cord, and other organs vulnerable. Most aortic arch repairs necessitate a period of systemic circulatory arrest combined with varying degrees of hypothermia to protect the brain and other vital organs. Prolonged hypothermic circulatory arrest (HCA) is associated with increased mortality, neurologic morbidity, and other complications. Cerebral perfusion (CP) techniques were developed to reduce these risks. Antegrade (ACP) and retrograde (RCP) techniques have been explored, and although definitive data are lacking, ACP appears to substantially reduce operative risk. In contemporary practice, nearly all aortic centers have shifted toward routinely using ACP to provide enhanced cerebral protection; although such protection may be provided in a unilateral fashion, provided the circle of Willis is complete, many centers, ours included, prefer to provide bilateral protection. That being said, HCA alone remains an acceptable approach when arch repair of limited duration (i.e., <30 minutes) is anticipated, and HCA with RCP continues to be favored by some centers because it can remove embolic debris and air, as well as potentially aiding cannulation approaches in cases of acute aortic dissection.
Over the last decade, we have changed the way we typically perform transverse aortic arch replacement. The changes include using ACP rather than RCP, placing the inflow cannula in the axillary or innominate artery instead of the femoral artery, and refining hypothermic targets: Mild (28.1°C to 34°C) or moderate hypothermia (20.1°C to 28°C), rather than profound hypothermia (14.1°C to 20°C). In addition, we now use trifurcated graft approaches rather than en bloc (island) approaches. Trifurcated grafts permit versatile arch reconstruction and employ the use of a single- or double-Y graft that is tailored to suit the patient’s anatomy as well as the extent of proposed repair.
To accommodate differences between the diameter of the replaced arch and the native distal aorta, we now use collared grafts.
To accommodate differences between the diameter of the replaced arch and the native distal aorta, we now use collared grafts.
Exposure and Cannulation
We perform aortic arch repair through a standard median sternotomy. Throughout the process of obtaining exposure, meticulous hemostasis is maintained to reduce problems with bleeding after the aortic repair. We currently favor using the innominate artery as the inflow site for cardiopulmonary bypass. When the innominate artery is not suitable for this purpose (due to aneurysm, dissection, or severe atherosclerotic disease involving the vessel), we use the right axillary artery for cannulation. In addition, when an aneurysm encroaches upon the sternum, cannulation on the axillary artery is preferred prior to sternal entry to avoid cutting directly into the aneurysm. In such cases, we access the axillary artery through an incision in the right deltopectoral groove. We separate the pectoralis major muscle fibers, divide the pectoralis minor muscle, and carefully mobilize the adjacent vein and cords of the brachial plexus.
When using the innominate artery as the inflow site, we administer intravenous heparin and apply a partially occluding clamp to the distal aspect of the vessel. After creating a longitudinal arteriotomy, we suture an 8-mm gelatin-sealed, woven polyester graft to the vessel (Fig. 12.2). Polypropylene suture is used for all anastomoses during repair unless otherwise noted. After the graft is carefully flushed and deaired, it is attached to the inflow line from the cardiopulmonary bypass circuit.
Other cannulas placed in preparation for cardiopulmonary bypass generally include a two-stage venous cannula placed via the right atrium, a left ventricular sump placed via the right superior pulmonary vein, and a coronary sinus retrograde cardioplegia cannula. After cardiopulmonary bypass is initiated, systemic cooling is begun to establish moderate hypothermia; our target temperature generally ranges between 23°C and 25°C nasopharyngeal temperature. If the patient has significant aortic valve regurgitation, it is often possible to cross-clamp the ascending aorta to prevent ventricular distension and enable delivery of cardioplegia while maintaining systemic flow and cooling through the innominate artery graft.
Reconstruction of Great Vessels
During the cooling phase, we begin the process of reconstructing the brachiocephalic vessels. We often find that it is easier to approach the LSCA after the arch aneurysm has
been decompressed than during the initial cooling period; under these circumstances, we typically address the LCCA first and reserve the LSCA for later. After the mid-portion of the LCCA is clamped and its base is ligated, the artery is divided and sutured end-to-end to the middle branch of a prefabricated trifurcated graft (Fig. 12.3). The graft has been stretched and trimmed to an appropriate length to avoid kinking. This anastomosis is performed while full flow from the cardiopulmonary bypass circuit is maintained.
been decompressed than during the initial cooling period; under these circumstances, we typically address the LCCA first and reserve the LSCA for later. After the mid-portion of the LCCA is clamped and its base is ligated, the artery is divided and sutured end-to-end to the middle branch of a prefabricated trifurcated graft (Fig. 12.3). The graft has been stretched and trimmed to an appropriate length to avoid kinking. This anastomosis is performed while full flow from the cardiopulmonary bypass circuit is maintained.
After the LCCA anastomosis is completed and the desired degree of hypothermia has been reached, systemic HCA is established. The proximal aspect of the innominate artery is occluded with a snare, and flow from the pump is reduced to approximately 10 mL/kg/min. The ascending aorta and transverse aortic arch are then opened. A balloon-tipped cannula is connected to a Y-limb from the inflow tubing and placed in the proximal aspect of the trifurcated graft to provide perfusion to the LCCA (Fig. 12.4). After the LSCA is transected, the first branch of the trifurcated graft is trimmed to
appropriate length and sutured end-to-end to the vessel. This branch of the graft is deaired and its clamp is removed, thereby enabling antegrade perfusion to the LSCA. When the left vertebral artery is anomalous and arises directly off the transverse aortic arch, we reimplant it by either transposing it end-to-side to the LCCA or directly onto the graft.
appropriate length and sutured end-to-end to the vessel. This branch of the graft is deaired and its clamp is removed, thereby enabling antegrade perfusion to the LSCA. When the left vertebral artery is anomalous and arises directly off the transverse aortic arch, we reimplant it by either transposing it end-to-side to the LCCA or directly onto the graft.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree