Aortic Arch and Vascular Anomalies
David S. Ezon
Daniel J. Penny
Introduction
It can be challenging to develop an adequate understanding of the three-dimensional interrelationships of the thoracic vessels and their embryologic predecessors. We encourage the reader to use the three-dimensional models which accompany this chapter to understand the normal development of the great arteries and how each lesion develops. The models are theoretical, based on the Rathke diagram and Edwards’ hypothetical double arch (1,2).
Classically, anomalies of the great arteries have been described with the aid of the totipotential diagram, a two-dimensional depiction of the great arteries and their branches. The totipotential diagrams are depicted for each lesion discussed, but the chapter will focus on the three-dimensional models to elucidate the anatomy.
While aortic arch anomalies have been documented as early as the 18th century (3), much remains unknown about these lesions. While some anomalies of the aortic arch effect as many as 1% of the population, others are quite rare. In the absence of a vascular ring, few result in symptoms, leading to underestimation of their prevalence. Though the anatomy of most lesions has been well documented (1), the proposed development of each lesion has largely remained theoretical. It is only recently that we have been able to document the embryologic underpinnings of aortic arch development (4) and in the absence of direct visualization of the development of the many abnormalities, it is not possible to be definitive about their exact origins.
Normal Anatomy
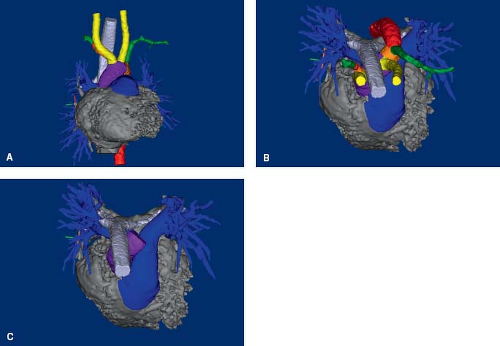
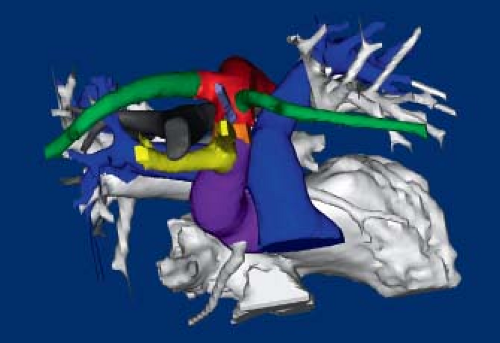
The normal aorta extends superiorly from the center of the heart, posterior to the pulmonary trunk (Fig. 33.1; Online 3-D Model 33.1). Because the ventricular outflow tracts cross each other, the aortic valve is to the right of the pulmonary valve. The ascending aorta continues as the transverse arch, which courses almost directly posterior, and only slightly leftward, abutting the left side of the trachea, and coursing over the left mainstem bronchus (Fig. 33.1). It then turns downward to continue as the descending thoracic aorta just leftward and anterior to the spine. The arterial duct, extending from the origin of the left pulmonary artery, inserts into the aortic
isthmus—the junction of the transverse and descending aorta. The transverse aortic arch most commonly gives rise to three branches. The first branch is the brachiocephalic artery, which courses rightward and superiorly for a short distance before dividing into the right subclavian artery and right common carotid artery. The right subclavian artery proceeds directly rightward toward the right arm, while the right common carotid artery proceeds superiorly and slightly rightward, toward the right side of the neck. The second branch of the aortic arch is the left common carotid artery, which proceeds superiorly and slightly leftward, toward the left side of the neck. The third branch is the left subclavian artery, which proceeds superiorly for a short distance before making a sharp turn leftward to continue directly toward the left arm. The subclavian arteries give rise to two important branches at their proximal end. The vertebral arteries arise from the superior aspect of the subclavian artery and proceed superiorly toward the head. The internal thoracic arteries (mammary arteries) proceed directly inferiorly along the ipsilateral side of the sternum, and connect with the anterior intercostal arteries. The descending thoracic aorta gives rise to the posterior intercostal arteries at each vertebral level, which connect to the corresponding anterior intercostal arteries.
isthmus—the junction of the transverse and descending aorta. The transverse aortic arch most commonly gives rise to three branches. The first branch is the brachiocephalic artery, which courses rightward and superiorly for a short distance before dividing into the right subclavian artery and right common carotid artery. The right subclavian artery proceeds directly rightward toward the right arm, while the right common carotid artery proceeds superiorly and slightly rightward, toward the right side of the neck. The second branch of the aortic arch is the left common carotid artery, which proceeds superiorly and slightly leftward, toward the left side of the neck. The third branch is the left subclavian artery, which proceeds superiorly for a short distance before making a sharp turn leftward to continue directly toward the left arm. The subclavian arteries give rise to two important branches at their proximal end. The vertebral arteries arise from the superior aspect of the subclavian artery and proceed superiorly toward the head. The internal thoracic arteries (mammary arteries) proceed directly inferiorly along the ipsilateral side of the sternum, and connect with the anterior intercostal arteries. The descending thoracic aorta gives rise to the posterior intercostal arteries at each vertebral level, which connect to the corresponding anterior intercostal arteries.
In the neck, there are two pairs of arteries that proceed superiorly to insert into the circle of Willis. The vertebral arteries course along the right and left aspect of the spine, within the spinal column, before joining together to form the basilar artery which inserts into the posterior aspect of the circle of Willis. The common carotid arteries divide into the external carotid arteries, which supply the face and ear, and the internal carotid arteries, which insert into the anterolateral aspects of the circle of Willis, through which they communicate with the vertebral arteries. This connection is important during certain pathologic states as it allows for “steal” from the circle of Willis to supply an isolated subclavian artery via the vertebral artery (see below).
Ten percent of people have only two vessels arising from the aortic arch. The right brachiocephalic artery arises together with left common carotid artery, via a common brachiocephalic trunk. Ten percent of people have four vessels arising from the aortic arch. In addition to the usual blood vessels, the left vertebral artery arises directly from the aortic arch, between the left common carotid artery and left subclavian artery, instead of arising from the left subclavian artery. Both of these findings are considered normal variants, but must be kept in mind when evaluating patients for other, pathologic arch anomalies (5).
The pulmonary trunk arises anterior to the aorta and proceeds leftward and posteriorly, spiraling along the left aspect of the ascending aorta (see Fig. 33.1B, C; Online 3-D Model 33.1). It then branches into the right and left pulmonary arteries. The right pulmonary artery courses rightward, underneath the aortic arch, but remains anterior to the trachea and bronchus. The left pulmonary artery proceeds leftward and posteriorly, toward the midaxillary line. The arterial duct originates at the branching point, closer to the left pulmonary artery, and proceeds in an anterosuperior and leftward direction to insert into the proximal descending aorta, immediately distal to the aortic isthmus.
Normal Embryology
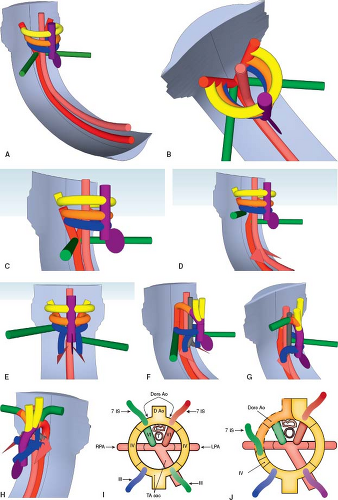
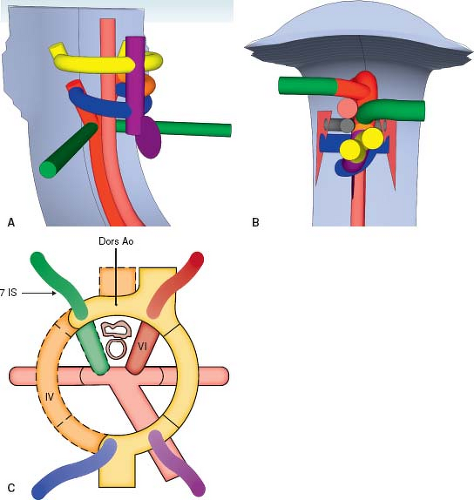
The developing heart forms within the pharyngeal mesoderm, in the region that develops into the neck, and migrates into the thorax over time. The heart tube is located ventral (anterior) to the pharyngeal pouches, a series of structures that give rise to the head and neck components (Fig. 33.2A, I; Online 3-D Model 33.2) (6). It is immediately ventral to the developing gut tube, which gives rise to the bronchial tree and lung buds. The truncus arteriosus is a channel that connects the ventricular mass to the aortic sac. It develops into the ventricular outflow tracts, and the aortic sac develops into the proximal great arteries. Dorsally (posteriorly) there are two parallel arteries, the dorsal aortae, which course caudally (inferiorly), on either side of the neural tube, before joining medially to form the descending aorta (Fig. 33.2B; Online 3-D Model 33.2). The aortic sac is connected to each dorsal aorta via a series of paired aortic arches that course along the left and right aspect of the gut tube in a ventral to dorsal (anterior to posterior) direction. One pair of aortic arches form for each pharyngeal pouch. The pairs of aortic arches do not all exist concurrently. Rather, they form sequentially before either regressing or developing into their final structures. These aortic arches give rise to the great arteries and their branches. Dorsal (posterior) to the dorsal aortae are the vertebral arteries, which course cranially (superiorly) toward the brain, eventually joining together to form the basilar artery which enters the posterior aspect of the circle of Willis, through which they will communicate with the internal carotid arteries. At each segmental level, the dorsal aortae give off intersegmental arteries that connect to the ipsilateral vertebral artery. The most important of the intersegmental arteries is the seventh intersegmental artery as it develops into the subclavian artery. This is why the vertebral arteries connect to the subclavian arteries in the mature embryo. The remainder of the intersegmental arteries regress.
The first pair of aortic arches form around the first pharyngeal pouch and give rise to a portion of the maxillary artery. The second pair of aortic arches form around the second pharyngeal pouch and give rise to the stapedial and hyoid arteries. Subsequently, the third, fourth, fifth, and sixth pairs of aortic arches are formed and then either regress completely or differentiate into their final form (Table 33.1). For the sake of understanding, Figure 33.2 depicts the third, fourth, and sixth arches concurrently. The fifth arch is not depicted because it usually regresses completely and does not contribute to the normal anatomy. The third aortic arches give rise to the common carotid arteries. The fourth aortic arches give rise to the segment of the aorta between the carotid artery and the subclavian artery. On the left side, it becomes part of the transverse aortic arch, while on the right side it becomes the proximal right subclavian artery (4).
The segment of the dorsal aortae between the third and fourth pair of aortic arches involutes, disconnecting the distal third aortic arches from the dorsal aortae (Fig. 33.2C, J; Online 3-D Model 33.2). The third aortic arches are therefore connected only at their proximal end, to the arterial sac, and are free to course cranially as the carotid arteries and eventually insert into the circle of Willis.
The region of the dorsal aorta distal to the seventh intersegmental artery regresses on the right side only, separating the right dorsal aorta along with the attached seventh intersegmental artery from the descending aorta (Fig. 33.2D; Online 3-D Model 33.2). On the left side, the dorsal aorta remains intact. This is what causes the arch to become a left aortic arch. Because the left dorsal aorta remains intact, blood can flow from the truncus arteriosus, through the fourth aortic arch to the left dorsal aorta and then to the descending aorta. On the right side, blood flows to the right third arch (right carotid artery) and to the fourth arch, continuing into the proximal right dorsal aorta and then right intersegmental artery (right subclavian artery). The blood is no longer able to course from the truncus arteriosus to the descending aorta via the right-sided arches, save for the sixth aortic arch, which will later regress (see below). The aortic sac gives rise to the ascending aorta and brachiocephalic artery. The portion of the aortic sac that forms the brachiocephalic artery connects to the proximal end of the right third aortic arch (right common carotid artery) and the proximal end of the seventh intersegmental artery via the right fourth aortic arch and right dorsal aorta.
The sixth aortic arches each give rise to a branch that enters the lung buds and form the right and left pulmonary arteries (Fig. 33.2E; Online 3-D Model 33.2). The distal right sixth aortic arch regresses, while the distal left sixth aortic arch develops into the arterial duct (Fig. 33.2G; Online 3-D Model 33.2). This is why the proximal end of the arterial duct arises from the proximal left pulmonary artery. The distal left sixth aortic arch inserts into the left dorsal aorta between the insertion point of the left fourth aortic arch and the origin of the left seventh intersegmental artery. Over time, the left seventh intersegmental artery migrates cranially such that it arises from the distal transverse aortic arch, immediately proximal to the insertion of the sixth aortic arch (Fig. 33.2F–H; Online 3-D Model 33.2). This is why the arterial duct usually inserts into the aorta immediately distal to the origin of the left subclavian artery.
TABLE 33.1 Theoretical Etiologies of Aortic Arch Anomalies | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Anomalies of the Aortic Arch
Arch sidedness is defined by which side of the trachea that the transverse aortic arch courses. A left aortic arch courses over the left mainstem bronchus, to the left of the trachea, while a right aortic arch courses over the right mainstem bronchus, to the right of the trachea. A double aortic arch is one with two transverse aortic arches, each coursing on either side of the trachea.
Some aortic arch anomalies result in a vascular ring or vascular sling, causing respiratory or gastrointestinal symptoms. A vascular ring is the presence of vascular structures that completely surround the trachea and esophagus. The ring can be formed by a patent vessel, an atretic vessel or its remnants (e.g., the arterial ligament). A vascular sling occurs when a branch pulmonary artery arises from the contralateral pulmonary artery and courses between the esophagus and the trachea, compressing them despite the absence of a true vascular ring.
It is important to note that in the normal state, the trachea abuts the right pulmonary artery on its anterior and right aspect and the aorta on its anterior and left aspect. There are no vessels coursing posterior to the trachea or esophagus. The normal arterial duct is remote from the trachea and does not border it. Therefore, there is no vascular ring surrounding the trachea and esophagus in the normal state.
Prompt diagnosis of aortic arch anomalies requires a high index of suspicion. Patients often present with prolonged histories of respiratory or gastrointestinal symptoms that have been misdiagnosed. They may go months without an accurate diagnosis, delaying adequate intervention (7). In one study, the majority of patients presented with wheezing (51%), followed by stridor (39%), pneumonia (25%), upper respiratory tract infection (24%), and respiratory distress (24%). Cough (22%) and respiratory cyanosis (19%) were also common. Respiratory arrest (8%), choking (7%), and bronchiolitis (7%) were not uncommon symptoms. Apnea, tracheomalacia, and aspiration were less common (≤3%). Patients also presented with gastrointestinal symptoms, including vomiting (19%), failure to gain weight appropriately (19%), and less commonly dysphagia (8%) and choking with feeds (5%). Murmurs were present in 10% of patients and heart failure in 8% (7).
Surgery can often be performed through a lateral thoracotomy (8). Surgical outcome is usually quite good for patients with symptomatic arch anomalies, with low surgical mortality and morbidity in otherwise well patients (7).
22q11 deletion plays an important role in aortic arch anomalies. The syndrome is characterized by maldevelopment of the third and fourth pharyngeal pouch, with a broad array of symptoms including aortic arch anomalies (particularly those related to the fourth aortic arch), conotruncal abnormalities, abnormal facies, cleft palate, parathyroid hypoplasia with secondary hypocalcemia, and thymic hypoplasia with secondary cellular immunodeficiency (9). Nearly one in five patients with conotruncal or aortic arch anomalies have 22q11 deletion (10). 22q11 deletion has been found in 24% of patients with isolated aortic arch branching and lateralization anomalies (11), and 50% of patients with interrupted aortic arch (10,12). Forty percent of patients with 22q11 deletion have aortic arch anomalies (9). It has been hypothesized that 22q11 deletion disrupts the neural crest cell migration integral to the development of the aortic arches, especially the fourth aortic arch (13). 22q11 deletion plays a significant role in perioperative care, and is associated with longer intensive care and hospital stay, longer duration of mechanical ventilation, and more frequent reoperation (14).
Mirror-Imaged Right Aortic Arch
Anatomy and Embryology
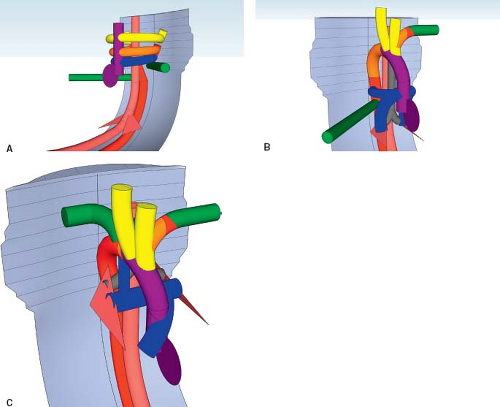
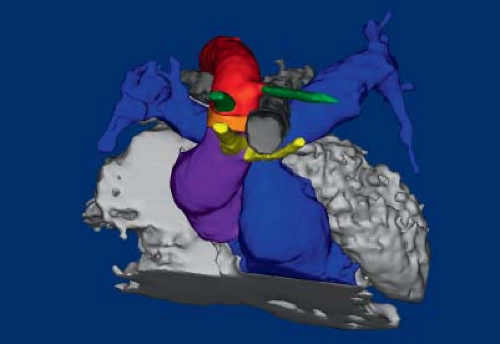
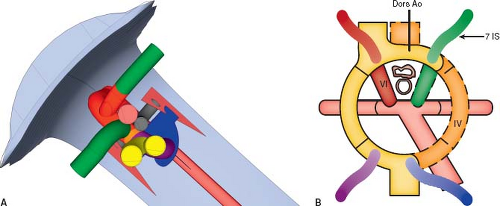
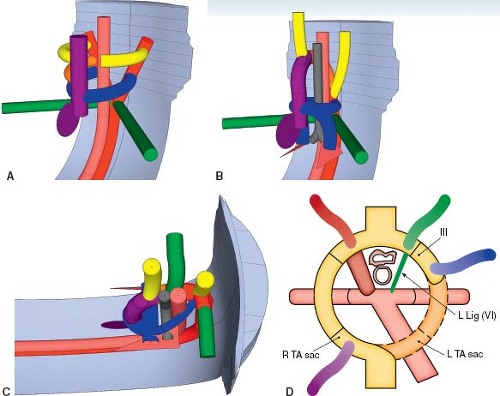
A right aortic arch is where the transverse aortic arch courses over the right mainstem bronchus. In a true mirror-imaged right aortic arch the arterial duct courses to the right of the trachea, and inserts into the aorta immediately distal to the aortic isthmus. The first branch of the aortic arch is a left brachiocephalic artery, followed by a right common carotid artery and a right subclavian artery (Fig. 33.3; Online 3-D Model 33.3). As with all right aortic arches, it is the left, rather than the right, dorsal aorta distal to the
left seventh intersegmental artery that regresses (Fig. 33.4A; Online 3-D Model 33.4). Thus, to reach the descending aorta, blood must flow through the right-sided arches, through the right dorsal aorta. In a true mirror-imaged right aortic arch, the distal right sixth aortic arch remains to form a right-sided arterial duct, while the distal left sixth aortic arch regresses, the opposite of what happens under normal circumstances (Fig. 33.4B). Therefore, the arterial duct extends from the proximal right pulmonary artery to the proximal descending aorta. With the involution of the distal left sixth aortic arch, the left seventh intersegmental artery remains connected to the truncus arteriosus only via the fourth aortic arch and left dorsal aorta. Thus, similar to what happens on the right side in the setting of a left aortic arch, over time the left seventh intersegmental artery (future left subclavian artery) migrates upward to join the left third aortic arch (future left carotid artery) and forms a left brachiocephalic artery. The right intersegmental artery remains attached directly to the right dorsal aorta. Therefore, in true mirror-imaged right aortic arch the first branch is a left-sided brachiocephalic artery, followed by the right common carotid artery, and then by the right subclavian artery and right-sided arterial duct (Fig. 33.4C).
left seventh intersegmental artery that regresses (Fig. 33.4A; Online 3-D Model 33.4). Thus, to reach the descending aorta, blood must flow through the right-sided arches, through the right dorsal aorta. In a true mirror-imaged right aortic arch, the distal right sixth aortic arch remains to form a right-sided arterial duct, while the distal left sixth aortic arch regresses, the opposite of what happens under normal circumstances (Fig. 33.4B). Therefore, the arterial duct extends from the proximal right pulmonary artery to the proximal descending aorta. With the involution of the distal left sixth aortic arch, the left seventh intersegmental artery remains connected to the truncus arteriosus only via the fourth aortic arch and left dorsal aorta. Thus, similar to what happens on the right side in the setting of a left aortic arch, over time the left seventh intersegmental artery (future left subclavian artery) migrates upward to join the left third aortic arch (future left carotid artery) and forms a left brachiocephalic artery. The right intersegmental artery remains attached directly to the right dorsal aorta. Therefore, in true mirror-imaged right aortic arch the first branch is a left-sided brachiocephalic artery, followed by the right common carotid artery, and then by the right subclavian artery and right-sided arterial duct (Fig. 33.4C).
More commonly, a right aortic arch is associated with an arterial duct that courses to the left of the trachea (15). This occurs because the left distal sixth aortic arch remains patent, while the right distal sixth aortic arch regresses (Fig. 33.5; Online 3-D Model 33.5). In this circumstance, the arterial duct usually inserts into the origin of the left brachiocephalic artery, rather than the proximal descending aorta. The trachea and esophagus are bound by the ascending aorta anteriorly, the transverse aorta to the right, and the descending aorta posteriorly. Because the arterial duct inserts into the base of the brachiocephalic artery, it remains anterior and therefore does not bound the left side of the trachea and esophagus, and no vascular ring is formed. However, at times the arterial duct does insert into the proximal descending aorta, in which case it will course posteriorly and border the left aspect of the trachea and esophagus, creating a vascular ring. Note the fact that in this situation a ring is formed because the arterial duct is located on the side contralateral to that of the transverse aortic arch, thereby completing the ring.
Epidemiology and Etiology
The prevalence of right aortic arch has been estimated at about 0.1%, based on necropsy and radiologic studies (16). The exact cause of right aortic arch has not yet been discovered. It is known that 22q11 deletion syndromes are associated with a right aortic arch, even in the absence of intracardiac disease (10). One proposed mechanism is that migration of neural crest cells into the aortic arch is impaired (17). Another is that the hemodynamics of blood flow from the outflow tracts causes the normal fetus to be prone to develop a left aortic arch. Lesions like tetralogy of Fallot or common arterial trunk disrupt the normal left-sided laminar flow such that the hemodynamics induce a right aortic arch to develop. Thus, fetuses with these lesions are at increased risk of developing a right aortic arch, even in the absence of
22q11 deletion (15,18). Interrupted aortic arch is also associated with 22q11 deletion, but it is not associated with a right ventricular outflow tract anomalies, and nearly always occurs in the setting of a left aortic arch, further supporting the idea that the fetal hemodynamics affect arch sidedness (15,19). Others have proposed mechanisms beyond genetics, including abnormal body folding (20,21).
22q11 deletion (15,18). Interrupted aortic arch is also associated with 22q11 deletion, but it is not associated with a right ventricular outflow tract anomalies, and nearly always occurs in the setting of a left aortic arch, further supporting the idea that the fetal hemodynamics affect arch sidedness (15,19). Others have proposed mechanisms beyond genetics, including abnormal body folding (20,21).
Associated Congenital Heart Disease
Up to 98% of patients with mirror-imaged right aortic arches have associated congenital heart disease (1,16), most commonly tetralogy of Fallot with or without pulmonary atresia (79%), and common arterial trunk (15,16,22,23). Twenty-five percent of patients with tetralogy of Fallot and between 35% and 60% of persons with common arterial trunk have a right aortic arch (1,16). Tricuspid atresia, particularly tricuspid atresia with normally related great arteries, pulmonary stenosis and ventricular septal defects may also occur (16). Right aortic arch is also associated with heterotaxy syndromes (16). It is common in persons with mirror-imaged dextrocardia with associated mirror-imaged abdominal viscera. However, in the absence of intracardiac disease, it has a lower prevalence in persons with dextrocardia but normally arranged abdominal viscera (16). On the other hand, only 4% to 5% of patients with transposition of the great arteries have a right aortic arch (16).
Persons with a right aortic arch and no associated cardiac disease usually have anomalous arch branching or a vascular ring (15). Those patients with a right aortic arch with mirror-imaged branching and no associated intracardiac disease frequently have stenosis or atresia of the left pulmonary artery (15). In one study of 11 patients with a right aortic arch with mirror-imaged branching but no associated intracardiac disease, 4 had associated left pulmonary artery stenosis or atresia, and 6 had a vascular ring due to an arterial duct/ligament coursing from the proximal left pulmonary artery to the descending aorta. Only one person studied had no associated vascular anomaly.
Clinical Manifestations
In the absence of a vascular ring or an associated cardiovascular anomaly, right aortic arch with mirror-imaged branching does not usually cause symptoms. However, most persons with a right aortic arch do have associated lesions that would cause them to present. Despite the lack of symptoms, the presence of a right aortic arch has several important clinical implications. It is critical that the arch sidedness is delineated prior to placing a Blalock–Taussig–Thomas shunt, because it may affect surgical management, including on which side the thoracotomy should be performed (22,24). Right aortic arch is also important in the management of infants with esophageal atresia and tracheoesophageal
fistulas. The prevalence of right aortic arch is increased in this population, up to 5% in one study (25,26,27,28). Approaching the esophagus through a thoracotomy on the same side as the transverse aortic arch may impair surgical access. Even with full knowledge of arch sidedness, right aortic arch is associated with more than twice the risk of surgical complications (26).
fistulas. The prevalence of right aortic arch is increased in this population, up to 5% in one study (25,26,27,28). Approaching the esophagus through a thoracotomy on the same side as the transverse aortic arch may impair surgical access. Even with full knowledge of arch sidedness, right aortic arch is associated with more than twice the risk of surgical complications (26).
Diagnostic Findings
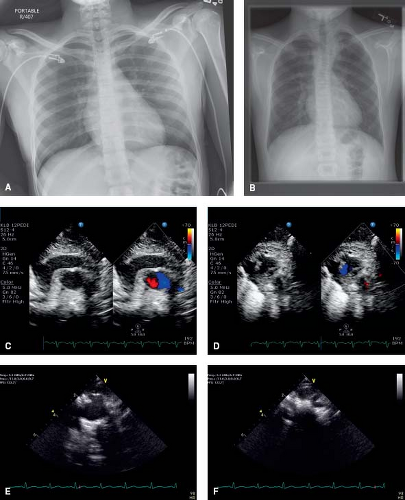
Chest x-rays of patients with a right aortic arch demonstrate the aortic knob located on the right side, rather than the left side of the chest (Fig. 33.6A, B). The diagnosis can be made on echocardiogram with reasonable assurance. From suprasternal notch view, starting with the aorta in cross section, a sweep superiorly will demonstrate the aortic arch branching pattern (Fig. 33.6C–F; Videos 33.1 and 33.2). In normal circumstances, the brachiocephalic artery is seen coursing rightward. Then, the left common carotid artery is visualized originating from the aortic arch and coursing leftward. Finally, the right brachiocephalic artery is seen dividing into the right subclavian artery and right common carotid artery. In the normal, left-sided aortic arch, the first branch of the aortic arch is the brachiocephalic artery, and it courses to the contralateral side of the arch. In a right aortic arch with
mirror-imaged branching, the first vessel is the left-sided brachiocephalic artery. Thus, the first vessel seen courses leftward and then divides. Finally, right aortic arches with aberrant subclavian arteries will not demonstrate the expected branching of the first blood vessel. These patients can be hard to differentiate from patients with a double aortic arch with atresia of the left arch between the left common carotid artery and left subclavian artery. Another clue to the arch sidedness on echocardiography is which side of the trachea the aortic arch is located. By turning the transducer 90 degrees, so the aorta is elongated and sweeping from right to left, one can track where the aorta is in reference to the trachea. CT and MRI are both excellent methods to delineate the aortic arch anatomy (29,30).
mirror-imaged branching, the first vessel is the left-sided brachiocephalic artery. Thus, the first vessel seen courses leftward and then divides. Finally, right aortic arches with aberrant subclavian arteries will not demonstrate the expected branching of the first blood vessel. These patients can be hard to differentiate from patients with a double aortic arch with atresia of the left arch between the left common carotid artery and left subclavian artery. Another clue to the arch sidedness on echocardiography is which side of the trachea the aortic arch is located. By turning the transducer 90 degrees, so the aorta is elongated and sweeping from right to left, one can track where the aorta is in reference to the trachea. CT and MRI are both excellent methods to delineate the aortic arch anatomy (29,30).
Aberrant Origin of the Subclavian Artery
Anatomy and Embryology
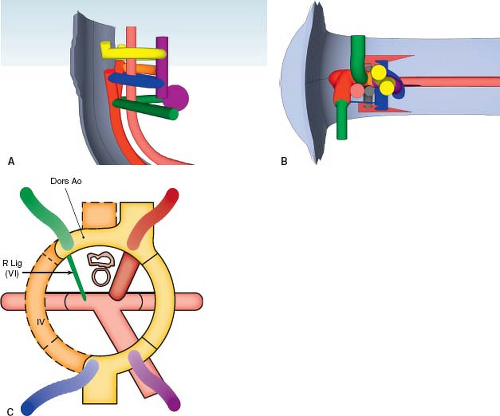
Left Aortic Arch with a Retroesophageal Right Subclavian Artery
A retroesophageal right subclavian artery originates from the proximal descending aorta, distal to the takeoff of the left subclavian artery, and courses rightward behind the esophagus (Fig. 33.7; Online 3-D Model 33.6). This occurs when the right fourth aortic arch regresses, so the right subclavian artery loses its connection to the aortic sac (Fig. 33.8; Online 3-D Model 33.7). The
distal right dorsal aorta remains intact, so the subclavian artery remains attached to the proximal descending aorta. Thus, instead of originating at the beginning of the aortic arch along with the right common carotid artery, it originates at the distal aortic arch, distal to the other aortic arch branches. While the right subclavian artery courses posterior to the esophagus, there is no vessel on the right side of the esophagus and trachea, so there is no vascular ring present. Rarely, the aberrant right subclavian artery can course between the trachea and esophagus, or even anterior to the trachea (2). Twenty percent of patients have a bicarotid trunk, with the right and left common carotid arteries arising together. In 14% of patients, the right vertebral artery arises from the right carotid artery, rather than the aberrant right subclavian artery, such that the first aortic arch branch divides, mimicking a brachiocephalic artery and obscuring the diagnosis (31).
distal right dorsal aorta remains intact, so the subclavian artery remains attached to the proximal descending aorta. Thus, instead of originating at the beginning of the aortic arch along with the right common carotid artery, it originates at the distal aortic arch, distal to the other aortic arch branches. While the right subclavian artery courses posterior to the esophagus, there is no vessel on the right side of the esophagus and trachea, so there is no vascular ring present. Rarely, the aberrant right subclavian artery can course between the trachea and esophagus, or even anterior to the trachea (2). Twenty percent of patients have a bicarotid trunk, with the right and left common carotid arteries arising together. In 14% of patients, the right vertebral artery arises from the right carotid artery, rather than the aberrant right subclavian artery, such that the first aortic arch branch divides, mimicking a brachiocephalic artery and obscuring the diagnosis (31).
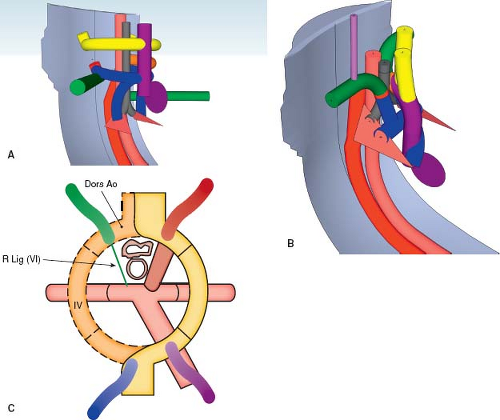
Right Aortic Arch with a Retroesophageal Left Subclavian Artery
Similar to its counterpart in the setting of a left aortic arch, a retroesophageal left subclavian artery in the setting of a right aortic arch is present when the left subclavian artery originates distal to the takeoff of right subclavian artery and courses leftward behind the esophagus, instead of arising from left brachiocephalic artery as expected (Figs. 33.9 and 33.10; Online 3-D Models 33.8 and 33.9). As with all right aortic arches, the distal right dorsal aorta remains patent. However, unlike most right aortic arches, in this setting the distal dorsal left aorta also remains and the left fourth aortic arch regresses. As well, the distal left sixth aortic arch involutes, while the distal right sixth aortic arch remains patent, yielding a right arterial duct, rather than a left arterial duct. Thus, the left seventh
intersegmental artery remains attached only to the dorsal aorta, because both the left fourth and sixth aortic arches have regressed. Therefore, the left subclavian artery originates from the aortic arch distal to the right subclavian artery, at the proximal descending aorta.
intersegmental artery remains attached only to the dorsal aorta, because both the left fourth and sixth aortic arches have regressed. Therefore, the left subclavian artery originates from the aortic arch distal to the right subclavian artery, at the proximal descending aorta.
Epidemiology and Etiology
A left aortic arch with an aberrant right subclavian artery is the most frequent aortic arch anomaly, effecting 0.4% to 2% of the population (1,32,33). A right aortic arch with an aberrant left subclavian artery is much less common, with an incidence of 0.1%. While the overall incidence is lower, patients with a right aortic arch have a 25% risk of having an aberrant left subclavian artery (32). Because the lesion is usually asymptomatic, the actual prevalence may be much higher (32). Patients with trisomy 21 and 22q11 deletion are at increased risk for aberrant subclavian arteries (32,34). The risk of aberrant subclavian arteries appears to be higher in Turner syndrome as well, with one study reporting an 8% prevalence (35).
Associated Congenital Heart Disease
One study found that 68% of patients with a left aortic arch and an aberrant right subclavian artery, and 75% of patients with a right aortic arch and an aberrant left subclavian had associated cardiac disease (32). However, the study was potentially prone to selection bias given that all subjects presented for echocardiograms. Earlier reports based on necropsy and angiography have demonstrated that right aortic arch with aberrant left subclavian artery has a low association with intracardiac disease (16). The true prevalence of congenital heart disease associated with aberrant subclavian arteries remains unknown. Patients with a right aortic arch were twice as likely to have an associated conotruncal anomaly, including tetralogy of Fallot and common arterial trunk (36% vs. 18%), and three times as likely to have an associated ventricular septal defect (35% vs. 11%). Patients with a left aortic arch and aberrant right subclavian artery were more likely to have left-sided obstructive lesions, including hypoplastic left heart syndrome and coarctation of the aorta, along with atrioventricular septal defects, though other studies have found the prevalence of left-sided obstructive lesions associated with aberrant subclavian artery to be low (34). Of all patients with tetralogy of Fallot studied, anomalous subclavian artery was more likely in those with a right aortic arch (16% vs. 5%). Similarly, of all persons with a common arterial trunk, anomalous subclavian artery was much more likely in those with a right aortic arch (30% vs. 3%). Coarctation of the aorta has been associated with aberrant right subclavian artery in 1% of cases (34,36). The aberrant right subclavian artery may be proximal or distal to the coarctation. It may impact the incidence of complications during repair, including the risk of paraplegia (37). Other studies have reported cases of right aortic arch with aberrant left subclavian artery in association with coarctation of the aorta and interruption of the aortic arch (22,38).
Clinical Manifestations
Aberrant subclavian arteries in the absence of a vascular ring are rarely associated with symptoms (16). Cases of dysphagia secondary to esophageal compression by a tortuous or ectatic right subclavian artery have been reported (1,16). Still, it is clinically important to know whether there exists an aberrant right subclavian artery before performing catheter-based angiography of the heart and coronaries because accessing the coronaries can be difficult via an aberrant subclavian artery (29). An aberrant right subclavian artery can also affect the signs associated with coarctation of the aorta. Should the aberrant right subclavian artery arise distal to the coarctation, there may not be a blood pressure gradient between the right arm and the lower extremities, providing false reassurance to the clinician. Both blood pressure and pulsation quality are likely to be depressed in the right arm. Given the increased incidence of aberrant right subclavian artery in coarctation of the aorta (34,36) the clinician should make a deliberate effort to assess for differences in the pulse and blood pressure not only between the upper and lower extremities, but also between the right and left upper extremities. Coarctation associated with an aberrant right subclavian artery that arises distal to the area of stenosis may demonstrate unilateral left-sided rib notching (29). Rarely, aortic dissection in the elderly has been associated with isolated aberrant right subclavian artery (39).
Diagnostic Findings
Diagnosis of an aberrant subclavian artery can be made with echocardiography by visualizing the absence of branching of the first branch of the aortic arch on suprasternal short-axis sweep, indicating the absence of a brachiocephalic artery. The first branch is the common carotid artery of the side contralateral to that of the arch sidedness (e.g., in a left aortic arch, the first branch is the right common carotid artery). As the sweep proceeds, the common carotid artery and subclavian artery ipsilateral to the arch can be visualized (e.g., in a left aortic arch, these are the left common carotid artery and left subclavian artery). Often, as the sweep continues, the aberrant subclavian artery can be visualized as a pulsatile structure extending superiorly from the descending aorta to the contralateral side. MRI and CT are also able to identify aberrant subclavian arteries (22). Chest x-ray may indicate the presence of an aberrant subclavian artery. The trachea may bow anteriorly on a lateral chest x-ray, simulating a mediastinal mass (29). In patients with a right aortic arch and aberrant left subclavian artery, there may be a prominent right-sided aortic knob that deviates the trachea to the left, and indents the right aspect of the trachea (29). In persons with a left aortic arch and an aberrant right subclavian artery, barium esophagram may show a posterior indentation angled obliquely from the inferior left to the superior right, in the vicinity of the aortic knob, due to an aberrant right subclavian artery heading superiorly and rightward from the proximal descending aorta (29). A right aortic arch with an aberrant left subclavian artery would similarly form an oblique indentation from the right inferior aspect to the left upper aspect of the esophagus.
Management and Outcome
Given the infrequency of associated symptoms, aberrant subclavian arteries in isolation do not require intervention.
Aberrant Origin of the Subclavian Artery with Associated Diverticulum of Kommerell
Anatomy and Embryology
Left Aortic Arch with a Right Retroesophageal Diverticulum of Kommerell
A right retroesophageal diverticulum of Kommerell is a protrusion from the proximal descending aorta that is directed rightward, behind the esophagus. The diverticulum connects to the right subclavian artery and right-sided arterial ligament. The etiology of a right retroesophageal diverticulum of Kommerell is similar to that of a retroesophageal right subclavian artery. During development, the right fourth aortic arch regresses, and the distal right dorsal
aorta remains, unlike in normal state (Fig. 33.11A, C; Online 3-D Model 33.10). The regression of the right fourth arch separates the right seventh intersegmental artery (future subclavian artery) from the proximal aorta, and the retention of the distal right dorsal aorta keeps the seventh intersegmental artery in continuity with the posterior arch, thus forming a left aortic arch with an aberrant origin of the right subclavian artery. However, in the setting of a diverticulum of Kommerell, the distal right sixth arch, which forms a right arterial duct, also remains. Because the arterial duct remained, during development there is much flow directed through the arterial duct to the retroesophageal portion of the distal dorsal aorta, causing it to dilate to a size similar to that of the descending aorta (Fig. 33.11B). The seventh intersegmental artery (right subclavian artery) receives the usual amount of blood flow and therefore maintains its normal caliber. Thus, there is a sharp taper at the junction of the diverticulum, the right subclavian artery, and the arterial duct. When the arterial duct constricts postnatally to form the arterial ligament, its presence may not be apparent by routine imaging. The fact that a diverticulum is present, however, indicates the existence of an arterial duct or arterial ligament. The vessels form a vascular ring because the ascending aorta is anterior to the trachea and esophagus, the transverse aortic arch is on the left side, the diverticulum is posterior to the esophagus, and the arterial ligament on the right side.
aorta remains, unlike in normal state (Fig. 33.11A, C; Online 3-D Model 33.10). The regression of the right fourth arch separates the right seventh intersegmental artery (future subclavian artery) from the proximal aorta, and the retention of the distal right dorsal aorta keeps the seventh intersegmental artery in continuity with the posterior arch, thus forming a left aortic arch with an aberrant origin of the right subclavian artery. However, in the setting of a diverticulum of Kommerell, the distal right sixth arch, which forms a right arterial duct, also remains. Because the arterial duct remained, during development there is much flow directed through the arterial duct to the retroesophageal portion of the distal dorsal aorta, causing it to dilate to a size similar to that of the descending aorta (Fig. 33.11B). The seventh intersegmental artery (right subclavian artery) receives the usual amount of blood flow and therefore maintains its normal caliber. Thus, there is a sharp taper at the junction of the diverticulum, the right subclavian artery, and the arterial duct. When the arterial duct constricts postnatally to form the arterial ligament, its presence may not be apparent by routine imaging. The fact that a diverticulum is present, however, indicates the existence of an arterial duct or arterial ligament. The vessels form a vascular ring because the ascending aorta is anterior to the trachea and esophagus, the transverse aortic arch is on the left side, the diverticulum is posterior to the esophagus, and the arterial ligament on the right side.
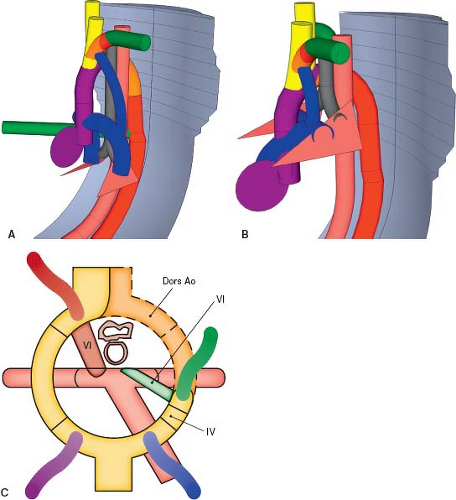
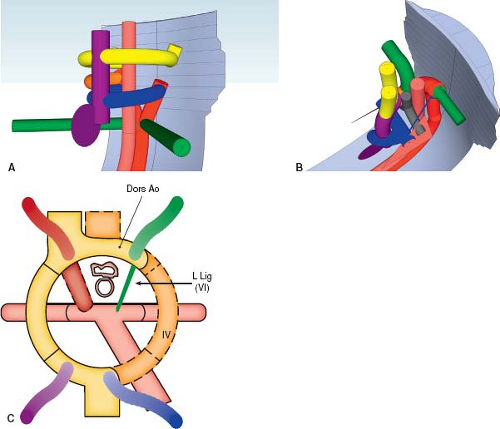
Right Aortic Arch with a Left Retroesophageal Diverticulum of Kommerell
A left retroesophageal diverticulum of Kommerell forms in a manner similar to its counterpart on the right, but with each developmental event occurring on the opposite side (Fig. 33.12; Online 3-D Model 33.11). The right fourth aortic arch and right distal dorsal aorta remain patent, causing a right aortic arch to form. Unlike in the usual development of a right aortic arch, however, the left fourth arch regresses and the left distal dorsal aorta remains patent, causing the left subclavian artery to originate aberrantly from the proximal descending aorta (Fig. 33.13A, C; Online 3-D Model 33.12). Additionally, the sixth aortic arch on the left remains patent, forming an arterial duct that extends from the proximal left pulmonary artery to the left distal dorsal aorta, where the aberrant left subclavian artery arises. As with its counterpart in the setting of a left aortic arch, the proximal right subclavian artery dilates to form a diverticulum of Kommerell due to the presence of the arterial duct directing flow to the descending aorta via the proximal left subclavian artery, inducing the proximal left subclavian artery to dilate. Postnatally, when the arterial duct constricts to form the arterial ligament, the diverticulum persists and is an indicator to the presence of the left-sided arterial ligament. This is clinically important as it indicates the presence of a vascular ring (Fig. 33.13B). The trachea and esophagus are bound by the ascending aorta anteriorly, transverse aorta on the right, descending aorta and aberrant left subclavian artery posteriorly, and the arterial duct/arterial ligament on the left.
Epidemiology and Etiology
A right aortic arch with a left-sided arterial duct/ligament, usually in association with a diverticulum of Kommerell and an aberrant left subclavian has been described as being the second most common cause
of a vascular ring, after double aortic arch (7,8,40,41). One report has demonstrated that while a vascular ring is present, the diverticulum of Kommerell may be absent if there is concurrent tetralogy of Fallot because reduced flow across the arterial duct during development had limited the dilation of the proximal subclavian artery (42).
of a vascular ring, after double aortic arch (7,8,40,41). One report has demonstrated that while a vascular ring is present, the diverticulum of Kommerell may be absent if there is concurrent tetralogy of Fallot because reduced flow across the arterial duct during development had limited the dilation of the proximal subclavian artery (42).
Clinical Manifestations
While the diverticulum of Kommerell is a common cause of a vascular ring, most patients are asymptomatic or have only mild symptoms because the ring is usually relatively loose (16,40). When symptoms do develop, they are related to compression of the esophagus by the retroesophageal subclavian artery (16). Patients may present as an infant or toddler with dysphagia or inspiratory stridor (43). If the carotid arteries arise in close proximity to each other, the trachea and esophagus may become entrapped between the bifurcation of the carotid arteries and the posterior aberrant subclavian arteries, increasing the risk for symptoms (40,44). There is a known long-term risk of atherosclerosis and tortuosity of aberrant subclavian artery associated with a diverticulum of Kommerell, occurring in 5% of patients (33,40,41,45). Most of these patients remain asymptomatic. However some may develop dysphagia, dyspnea, stridor, wheeze, cough, recurrent lower respiratory tract infections, obstructive emphysema, or chest pain due to tracheoesophageal compression by a rigid, tortuous aberrant subclavian artery (46,47,48). There is also a long-term risk of aneurysm, occurring in up to 8% of patients (49). Nearly half of these patients suffered a rupture of the aneurysm, calling on some to recommend surgical intervention even in asymptomatic patients (49,50,51,52).
Diagnostic Features
Barium esophagram suggests a diverticulum of Kommerell if it demonstrates a posterior esophageal indentation on the side ipsilateral to the arch (29) (Fig. 33.14; Videos 33.3 and 33.4). This occurs because the aortic arch is pulled toward the esophagus by the arterial ligament, which is tethered to the left pulmonary artery (1,43,48). A right aortic arch with an aberrant subclavian artery and left-sided arterial duct causes the indentation on the right aspect (1,43). While the presence of indentations on both sides of the esophagus usually indicates a double aortic arch, an aberrant subclavian artery with a diverticulum of Kommerell can also cause bilateral indentation due to the impression of the aorta on one side and the arterial ligament on the other side (1,8,43). The indentation on the left side is usually lower than that on the right when the vascular ring is due to a diverticulum of Kommerell (43). Should the patient undergo an endoscopy, a pulsatile mass compressing the esophagus may be found. CT and MRI are able to delineate the anatomy well (48,53).
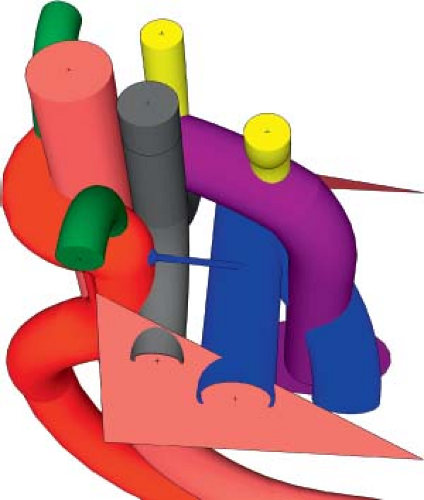
Right Aortic Arch with Retroesophageal Left Brachiocephalic Artery
There are rare hearts with a right aortic arch and a left brachiocephalic artery that arises distal to the right subclavian artery and courses behind the esophagus toward the left side (Fig. 33.15; Online 3-D Model 33.13). This occurs due to the regression of the truncoaortic sac at the junction with the left third aortic arch, as well as the regression of the fourth aortic arch (Fig. 33.16; Online 3-D Model 33.14). This regression of the truncoaortic sac’s connection to the proximal end of the left third aortic arch separates it from the proximal arterial trunk. At the same time, the left fourth aortic arch regresses and the distal right dorsal aorta remains patent, creating a right aortic arch. Also, the entire left dorsal aorta remains patent, such that the left third aortic arch is now connected at its distal end to the left dorsal aorta, which itself is connected to the left seventh intersegmental artery and the proximal descending aorta. As with aberrant left subclavian arteries, the involution of the left fourth aortic arch causes the left seventh intersegmental artery to be connected to the proximal descending aorta. The left sixth arch remains to form a left-sided arterial duct coursing between the proximal left pulmonary artery and the base of the aberrant
brachiocephalic artery, thereby forming a vascular ring. The excess flow through the proximal brachiocephalic artery from the arterial duct causes the proximal brachiocephalic artery to dilate and form a diverticulum of Kommerell. Unlike in the usual circumstances, here the diverticulum of Kommerell gives off the left common carotid artery in addition to the left subclavian artery. The vascular ring is formed by the ascending aorta and pulmonary artery anteriorly, the transverse aortic arch on the right, the diverticulum of Kommerell (proximal aberrant brachiocephalic artery) posteriorly, and the arterial duct on the left.
brachiocephalic artery, thereby forming a vascular ring. The excess flow through the proximal brachiocephalic artery from the arterial duct causes the proximal brachiocephalic artery to dilate and form a diverticulum of Kommerell. Unlike in the usual circumstances, here the diverticulum of Kommerell gives off the left common carotid artery in addition to the left subclavian artery. The vascular ring is formed by the ascending aorta and pulmonary artery anteriorly, the transverse aortic arch on the right, the diverticulum of Kommerell (proximal aberrant brachiocephalic artery) posteriorly, and the arterial duct on the left.
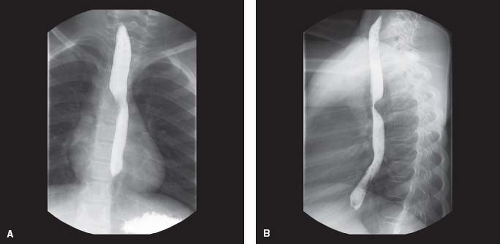 Figure 33.14 Barium esophagram of a patient with a right aortic arch and left retroesophageal diverticulum of Kommerell. A: Anterior view. B: Lateral view. |
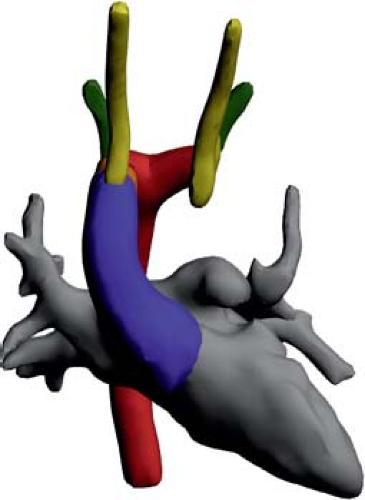 Figure 33.15 Rendition of a right aortic arch with retroesophageal left brachiocephalic artery based on a CT angiogram. |
Right aortic arch with an aberrant brachiocephalic artery is a very rare (54,55) and no cases have been reported in association with a left aortic arch. It has been reported in association with ventricular septal defect, tetralogy of Fallot (55,56), and coarctation of the aorta (57). Patients may present with the usual symptoms of a vascular ring, including respiratory symptoms and dysphagia, or may be asymptomatic, with the diagnosis made incidentally (56,58). On barium esophagram, they demonstrate a right-sided indentation from the right aortic arch, and a posterior indentation from the diverticulum of Kommerell (proximal aberrant brachiocephalic artery) (55,58). Echocardiography demonstrates the transverse arch to the right of the trachea and the left brachiocephalic artery arising from the proximal descending aorta, best imaged from the suprasternal window (59). Note that this is one of the few instances where the first common carotid artery arising from the aortic arch is ipsilateral to the transverse arch. MRI and CT are effective in delineating the anatomy (56,60), and demonstrate a diverticulum of Kommerell protruding from the proximal descending aorta and giving off the brachiocephalic artery.
Isolated Branches of the Aortic Arch
Anatomy and Embryology
Left Aortic Arch with Isolated Right Subclavian Artery
An isolated right subclavian artery is one that is disconnected from the aortic arch. It is connected only to the right-sided arterial duct. Closure of the arterial duct would eliminate any prograde flow to the subclavian artery, in which case, the subclavian artery would be supplied by retrograde flow from the circle of Willis. An isolated right subclavian artery develops when there is regression of the right fourth arch (Fig. 33.17; Online 3-D Model 33.15). Unlike in the setting of an aberrant right subclavian artery from the descending aorta, the right dorsal aorta also regresses, leaving the seventh intersegmental artery (the subclavian artery) attached only to the right sixth arch. The right sixth arch does not regress and instead forms a right-sided arterial duct, the sole source of prograde flow into the subclavian artery. The seventh intersegmental artery retains its attachment to the vertebral artery, thereby allowing retrograde flow from the circle of Willis to the subclavian artery via the vertebral artery or from the contralateral vertebral artery via the basilar artery.
Right Aortic Arch with Isolated Left Subclavian Artery
Patients may have a right aortic arch with an isolated left subclavian artery, supplied by prograde flow through a left-sided arterial duct or retrograde flow through the left vertebral artery. The right aortic arch is formed through the usual process, with regression of the left distal
dorsal aorta and retention of the right distal dorsal aorta. The left seventh intersegmental artery’s remaining connection to the arch is via the left fourth arch, which also regresses, isolating it from the arch. The left sixth arch, which forms the left arterial duct, connects the isolated left seventh intersegmental artery (future left subclavian artery) to the pulmonary artery, enabling prograde flow from the pulmonary artery to the left subclavian artery via the arterial duct. The seventh intersegmental artery retains its attachment to the vertebral artery, thereby allowing retrograde flow from the circle of Willis to the subclavian artery via the vertebral artery. Alternatively, the subclavian artery may be supplied by collateral vessels. Isolation of the subclavian artery does not usually cause a vascular ring. Rarely, the isolation may be incomplete, such that an atretic cord remains between the aortic arch and the isolated subclavian artery, with a diverticulum of Kommerell located posterior to the aorta. In this setting, a vascular ring is formed from the ascending aorta and pulmonary artery anteriorly, transverse arch on the right, diverticulum of Kommerell and the atretic portion between the aortic arch and the isolated subclavian artery posteriorly, and the arterial duct on the left (61,62).
dorsal aorta and retention of the right distal dorsal aorta. The left seventh intersegmental artery’s remaining connection to the arch is via the left fourth arch, which also regresses, isolating it from the arch. The left sixth arch, which forms the left arterial duct, connects the isolated left seventh intersegmental artery (future left subclavian artery) to the pulmonary artery, enabling prograde flow from the pulmonary artery to the left subclavian artery via the arterial duct. The seventh intersegmental artery retains its attachment to the vertebral artery, thereby allowing retrograde flow from the circle of Willis to the subclavian artery via the vertebral artery. Alternatively, the subclavian artery may be supplied by collateral vessels. Isolation of the subclavian artery does not usually cause a vascular ring. Rarely, the isolation may be incomplete, such that an atretic cord remains between the aortic arch and the isolated subclavian artery, with a diverticulum of Kommerell located posterior to the aorta. In this setting, a vascular ring is formed from the ascending aorta and pulmonary artery anteriorly, transverse arch on the right, diverticulum of Kommerell and the atretic portion between the aortic arch and the isolated subclavian artery posteriorly, and the arterial duct on the left (61,62).
Epidemiology and Etiology
Data on isolated subclavian arteries are sparse and come mainly from case reports (1,16,62). Isolated subclavian arteries occur very rarely, with one report finding an incidence of isolated left subclavian arteries in <1% of patients with a right aortic arch (63). Cases of left aortic arch with isolated right subclavian artery have also been reported, but much less frequently (16,64,65).
Associated Congenital Heart Disease
Clinical Manifestations
Isolated subclavian arteries can present with depressed pulse or decreased blood pressure in the ipsilateral upper extremity (16,61,66,67,68). Some patients develop neurologic symptoms because of a subclavian steal phenomenon, whereby blood flow in the circle of Willis is diverted to the subclavian artery via the vertebral artery, causing vertebrobasilar insufficiency. Patients may experience vertigo, headache, or syncope. Patients may also develop pain, weakness, paresthesias, or coldness in the affected arm from poor perfusion (61,69). Of note, subclavian steal syndrome may present in patients with normal arterial anatomy in adults with atherosclerosis causing obstruction to prograde subclavian blood flow (70). Most patients are asymptomatic. In a review of 30 patients, only 5 developed symptoms of ischemia of the affected limb, and 5 developed symptoms of vertebrobasilar insufficiency (61). Symptomatic patients presented in adulthood, though many patients had symptoms for more than a decade before being diagnosed, demonstrating the need for a high index of suspicion. In addition to subclavian steal syndrome, pulmonary steal syndrome has been reported (66,71), due to a persistent arterial duct. When the pulmonary vascular resistance decreases in the newborn period, retrograde flow in the ipsilateral vertebral artery is directed mainly into the arterial duct toward the pulmonary vascular bed, because the pulmonary resistance is less than that of the arm. The vertebral flow only supplies the proximal left subclavian artery. These patients may develop signs both of vertebrobasilar insufficiency and decreased perfusion to the affected limb (71).
Diagnostic Findings
The diagnosis of an aberrant subclavian artery should be considered when there is a difference in pulse intensity and blood pressure between the upper extremities (16,66,67,68). Angiogram of the aorta demonstrates delayed opacification of the affected subclavian artery, with late filling via retrograde flow through the vertebral artery or collateral vessels (16,61,64,72). Selective injection of the ipsilateral vertebral artery may show flow through the arterial duct and into the pulmonary vascular bed, indicative of pulmonary steal syndrome (71). Echocardiogram may demonstrate to and fro flow at the main pulmonary artery due to retrograde flow from the vertebral artery (66). Isolated subclavian arteries can also be diagnosed by CT or MRI (69,73). Barium esophagram is not helpful as there is rarely a posterior diverticulum of Kommerell to cause an indentation.
Right Aortic Arch with Isolated Brachiocephalic Artery
Cases of isolation of the left brachiocephalic artery or left carotid artery have also been reported. In these patients, the brachiocephalic artery is supplied by mediastinal or vertebral collateral vessels and a left-sided arterial duct. It presents with depressed pulses and blood pressure in the left arm relative to the right arm. The vertebral arteries originate directly from the aortic arch (75,76,77).
Circumflex Aorta
Left Aortic Arch with a Right Descending Aorta and a Right Arterial Duct
Rarely, a left aortic arch may turn rightward after passing the trachea and esophagus, and descend on the right side of the trachea and esophagus before gradually returning to the left side to continue its descent toward the abdomen. It may occur concurrently with an aberrant right subclavian artery. The lesion forms if during development the left dorsal aorta migrated rightward, behind the esophagus (Fig. 33.18; Online 3-D Model 33.16). As well, the right
fourth aortic arch regressed and the right dorsal aorta remained so that the right seventh intersegmental artery (future right subclavian artery) originates aberrantly from the proximal descending aorta. Either the right or left distal sixth aortic arch may remain to form a right or left-sided arterial duct, respectively. If a right-sided arterial duct forms, a vascular ring is formed, with the ascending aorta anterior to the trachea, the transverse aortic arch bordering the left side of the trachea and esophagus, the transverse and proximal descending aorta bordering the posterior aspect of the esophagus, and the right-sided arterial duct or arterial ligament bordering the right side of the trachea and esophagus (16).
fourth aortic arch regressed and the right dorsal aorta remained so that the right seventh intersegmental artery (future right subclavian artery) originates aberrantly from the proximal descending aorta. Either the right or left distal sixth aortic arch may remain to form a right or left-sided arterial duct, respectively. If a right-sided arterial duct forms, a vascular ring is formed, with the ascending aorta anterior to the trachea, the transverse aortic arch bordering the left side of the trachea and esophagus, the transverse and proximal descending aorta bordering the posterior aspect of the esophagus, and the right-sided arterial duct or arterial ligament bordering the right side of the trachea and esophagus (16).
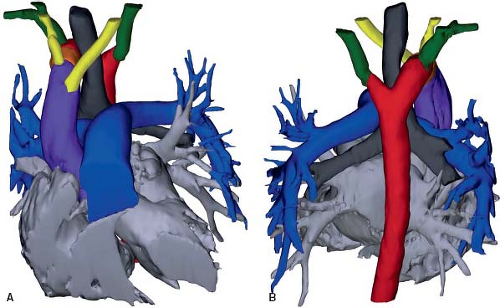
Right Aortic Arch with Left Descending Aorta and a Left Arterial Duct
Similar to its counterpart in the setting of a left aortic arch, a right aortic arch may turn leftward after passing the trachea and esophagus, to descend on the left side of the trachea and esophagus (Fig. 33.19; Online 3-D Models 33.17 and 33.18; Video 33.5). This occurs if the right dorsal aorta migrates leftward, behind the esophagus. The left distal aortic arch may regress, as would be expected for a right aortic arch. Alternatively, it may persist while the right fourth aortic arch regresses, causing the left seventh intersegmental artery to originate from the proximal descending aorta via the distal left dorsal aorta, to form an aberrant left subclavian artery. If the distal left sixth aortic arch remains, forming a left-sided arterial duct that inserts into the proximal descending aorta, a vascular ring is formed. The trachea and esophagus are bound by the ascending aorta anteriorly, transverse aorta to the right, and left arterial duct/ligament to the left. The posterior aspect of the esophagus is bordered by the transverse and proximal descending aorta, as it crosses from the right to the left side (16).
Epidemiology and Etiology
Associated Congenital Heart Disease
In one report, half of patients reviewed had an associated cardiac lesion (16). Patients may have severe hypoplasia of the retroesophageal portion of the transverse aortic arch. All of these patients had a right aortic arch with a left-sided arterial duct and an aberrant left subclavian artery from the descending aorta, at the site of ductal insertion. These patients presented with symptoms of arch obstruction and may be impossible to differentiate from interruption of the aortic arch without a CT or MRI (78,79).
Clinical Manifestations
Patients with a circumferential aortic arch may present early with signs of ductal-dependent aortic arch obstruction and it may be difficult to differentiate them from patients with an interruption of the aortic arch (78,79), or they may present late due to respiratory symptoms including recurrent infections and chronic cough, or dysphagia (16).
Diagnostic Findings
Chest x-ray findings may resemble that of a double aortic arch, with an aortic knob on both sides of the trachea, due to the transverse arch on one side, and the descending aorta on the other (80). The opacity may be confused for a mediastinal mass (80). Barium esophagram demonstrates a large, smooth, round, pulsatile impression on the posterior aspect of the aorta due to the transverse aortic arch. In one study, patients with a left circumferential aorta also had an impression on the left aspect of the esophagus (16). CT is diagnostic and can demonstrate the anatomy well. It may be difficult to differentiate a circumferential aortic arch with a hypoplastic transverse portion from an interruption of the aortic arch on echocardiogram, in which case a CT or MRI is warranted (78,79).
Management and Outcome
Because the vascular ring is relatively loose, most patients do not require treatment. If symptoms warrant surgical intervention, dividing the vascular ring alone may not be sufficient (81). The vascular ring should be approached from the right side so the surgeon can better access the arterial ligament (82). The patient may require arch reconstruction with resection of the retroesophageal transverse arch and either an arch advancement or an interposition graft between the ascending and descending aorta (78,81,82,83).
Double Aortic Arch
In a double aortic arch, the ascending aorta divides into two transverse aortic arches, each coursing on either side of the trachea, over each mainstem bronchus (Fig. 33.20; Online 3-D Models 33.19
and 33.20). The descending aorta is on the left side of the spine. Therefore, the right transverse arch courses posteriorly and leftward behind the esophagus, to insert into the descending aorta. The aortic arch branches are arranged symmetrically, with the right common carotid artery and right subclavian artery arising separately from the right transverse arch, and the left common carotid artery and left subclavian artery arising from the left transverse arch. The arterial duct may insert on either side, or be bilateral. Usually, it is left sided. It may insert into the proximal descending aorta, or into the left aortic arch (1,2).
and 33.20). The descending aorta is on the left side of the spine. Therefore, the right transverse arch courses posteriorly and leftward behind the esophagus, to insert into the descending aorta. The aortic arch branches are arranged symmetrically, with the right common carotid artery and right subclavian artery arising separately from the right transverse arch, and the left common carotid artery and left subclavian artery arising from the left transverse arch. The arterial duct may insert on either side, or be bilateral. Usually, it is left sided. It may insert into the proximal descending aorta, or into the left aortic arch (1,2).
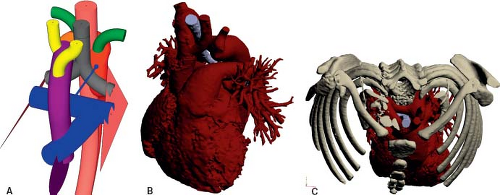 Figure 33.19 Right aortic arch with left descending aorta and a left arterial duct. A: Schematic model. B: Rendition based on a CT angiogram. C: Rendition based on a CT angiogram. |
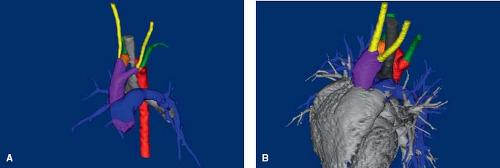 Figure 33.20 Double aortic arch. Renditions of a double aortic arch with two patent vessels (A) and with atresia of the middle segment of the left aortic arch (B) based on CT angiograms.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|