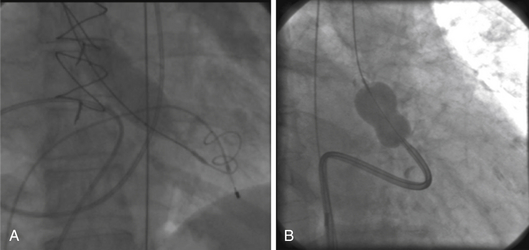
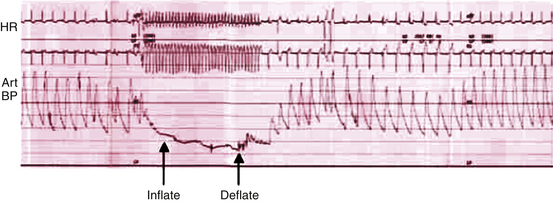
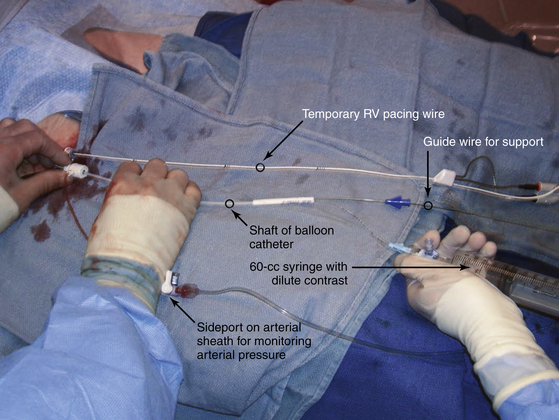
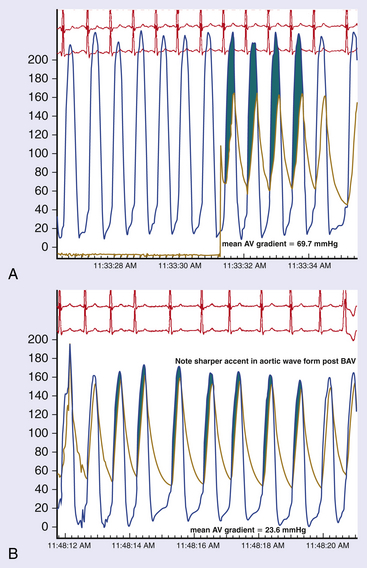
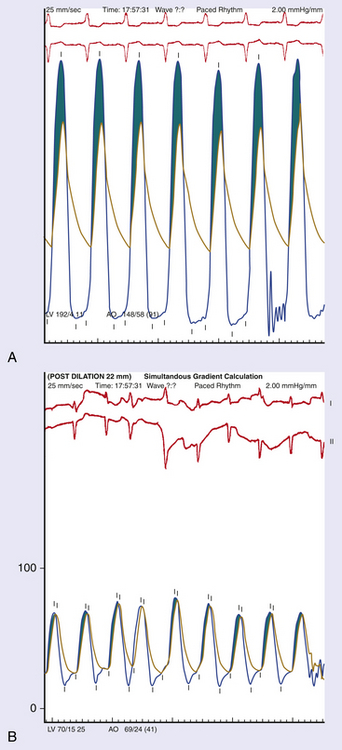
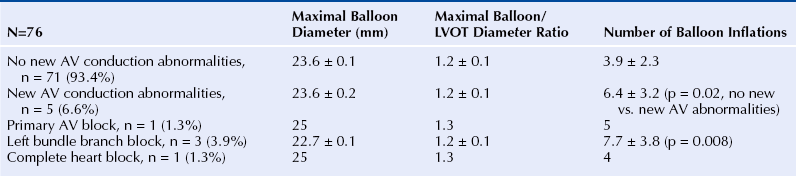
Chapter 5 Balloon aortic valvuloplasty (BAV) for severe aortic stenosis (AS), a common disease of the elderly, was introduced by Dr. Cribier in 1986.1 Initial experience demonstrated the technical feasibility, acceptable safety, and fairly modest improvement in valve areas. In spite of an only modest improvement in valve area, patients experienced significant symptomatic benefit, resulting in an initially enthusiastic embrace by interventional cardiologists. However, the subsequent recognition of high restenosis rates within a year of the procedure quickly tempered enthusiasm. Large series have demonstrated restenosis rates of 40% to 80% within 5 to 9 months and a failure to improve survival despite palliative benefits of BAV.2–5 The clinical lag in symptomatic recurrence extends the palliative benefit 6 to 9 months beyond hemodynamic restenosis. Therefore an overall quality-of-life (QOL) benefit after BAV may last up to between 1 and 1.5 years in elderly patients who are generally less concerned about durable efficacy.4 Calcific AS is the most common expression of primary valvular heart disease in adults. Moderate to severe AS is observed in 4.6% of patients older than 75 years of age.6 Surgical aortic valve replacement (AVR) is the preferred treatment strategy for patients of all age groups, although it has limitations in octogenarians and nonagenarians. Eight retrospective studies of patients over 80 years of age showed a pooled 30-day mortality rate of 9.2% for isolated AVR and 20.9% for combined AVR and coronary bypass grafting.7 Transcatheter AVR (TAVR) has expanded the option for valve replacement therapy in elderly patients who are at high risk for surgery because of comorbidities and frailty. Although TAVR offers a more durable improvement for the treatment of AS than BAV, BAV is being carried out with increasing frequency for a number of reasons. In the largest registry (i.e., National Heart, Lung, and Blood Institute [NHLBI]), outcomes of procedures performed in the late 1980s demonstrated the significant limitation in clinical benefit for younger patients in their late 70s, predominantly linked to restenosis. High complication rates were also reported in these early series (i.e., 25% in the NHLBI series; Table 5–1).4,8 TABLE 5–1 The reemergence of BAV since 2000 resulted from a more clear definition of its palliative role in high-risk patient subsets, its requirement for predilation in TAVR, and its utility in clarifying the role of AS in symptomatic patients with multiple disease states before TAVR. In addition, broader adoption has resulted from improvements in technique. Recent series have highlighted improved outcomes and reduced complications (Table 5–2).9 Calcific AS is characterized by increased leaflet thickness, calcification, and rigid immobility, generally in the absence of commissural fusion. Until recently, calcific AS was considered to be histopathologically degenerative or passive in origin. It is now recognized as a complex, active, cellular process with features of atherosclerosis and biomineralization similar to osteogenesis in bone formation.10 The effects of BAV on stenosed aortic valves are poorly understood, but several mechanisms appear likely.11 Primarily, balloon-induced fracturing of calcified nodules create hingepoints,12 and along with the creation of cleavage planes in collagenous stroma, improve leaflet flexibility and valve opening. Separation of fused leaflets is uncommon given its infrequent occurrence in nonrheumatic patients. Enhanced compliance or stretching of the adjacent annulus and calcified aortic root have also been suggested.13 Restenosis is generally believed to occur from scar tissue (cell-rich pattern of fibroblasts, capillaries, and inflammatory infiltrate) within small leaflet tears.14 Heterotopic ossification (bone formation) was reported to occur in another small series.15 On occasion, recoil of the elastic valve components may result in early valve renarrowing within hours to days. Current American College of Cardiology/American Heart Association (ACC/AHA) guidelines are listed in Box 5–1.16 The substantial increase in BAV volumes over the past 5 to 10 years has come about for two primary reasons. The first is based on a realization that an increasing population of elderly patients with comorbidities and who are at high risk or otherwise poorly suited for surgical AVR derive significant palliation. Multiple published experiences have shown a consistent reduction in New York Heart Association (NYHA) functional class, the preponderance improving from functional class III/VI to I/II.8,17–19 The demonstrated safety in serial BAVs for patients with recurrent restenosis extends the opportunity for achieving longer periods of enhanced QOL. In general, these authors like to see at least 6 months of benefit before performing a subsequent BAV. In cases where this time is shorter, underdilation on the previous intervention may offer the opportunity for more aggressive increments in balloon sizing. Although unproven, some authors have suggested a survival benefit as well.17,20 With improved technique, symptomatic AS patients who are not candidates for surgical AVR or TAVR should be offered palliative BAV in place of medical therapy alone. Second, the option for TAVR in poor–surgical-risk groups has had an explosive impact on the use of BAV that is not only essential for predilation, but has now been reported to successfully bridge patients to TAVR.21 Patients initially too unstable in one series underwent successful TAVR after a mean interval of 59 ± 57 days. However, the precise role for BAV as a bridge requires further study. Many patients with severe AS and symptoms have other comorbidities such as severe lung disease that may play a significant role in their symptom complex. A “diagnostic” BAV can be performed to evaluate the role AS is playing, and if it is significant, BAV can then be followed by TAVR. Candidates in the current “TAVR era” for stand-alone BAV in the adult cardiology practice at Minneapolis Heart Institute (MHI) are shown in Box 5–2. BAV can be carried out using two different approaches that are well-described in the literature.22–24 The most commonly used approach is the retrograde technique. The antegrade technique, which will be discussed in less detail, is predominantly indicated in patients with inadequate arterial access (Figure 5–1). Both right and left groins are prepped. The authors most commonly use just the right side for arterial and venous access. Six-French femoral arterial and 8F femoral venous sheaths are placed when the patient is under local anesthesia. Care is taken to document appropriate landmarks to ensure arterial access through the anterior wall of the common femoral artery. After confirming common femoral artery access with a contrast injection, the arterial access site is preclosed using two 6F ProGlide devices (Abbott Vascular, Abbott Park, Ill.).25 Sutures are deployed sequentially at 90-degree angles to each other at the 10 o’clock and 2 o’clock positions and draped to either side with Kelly clamps. A 10F to 12F sheath is then exchanged for the initially placed sheath, depending on valvuloplasty balloon type and size. Alternatively, a single 10F Prostar device (Abbott Vascular, Abbott Park, Ill.) can be used. Patients are then given 70 units/kg of unfractionated IV heparin, which is titrated to achieve an activated clotting time (ACT) of 250 to 300 seconds. Coronary angiography is performed, most importantly, to rule out critical stenosis of the left main coronary artery before proceeding with BAV. An unprotected critical left main coronary artery, if anatomically suitable, should be stented before performing BAV. Other severe lesions felt to be clinically relevant can be treated safely after BAV as a combined procedure. See the subsequent discussion on combined coronary stenting and BAV. Patients undergoing BAV will be heparinized before the aortic valve is crossed. Nevertheless, it is important to routinely wipe wires and flush catheters with heparinized saline every 2 minutes during attempted crossing. Although one’s choice of catheters for crossing the valve is somewhat personal, most operators use 6F Amplatz left (AL; Cordis Corporation; Bridgewater, N.J.) diagnostic catheters to best achieve a coaxial trajectory with a straight wire to access the left ventricle. A preferred choice has been an AL-1; however, an AL-2 configuration, especially in patients with a horizontal root and vertical valve, may be preferred.24 The authors prefer to attempt crossing in a 20-degree to 40-degree left anterior oblique projection. A brief cine run for two to three cardiac cycles without contrast is first captured for defining the precise location of systolic leaflet separation. The catheter is then positioned within the systolic jet, which is confirmed fluoroscopically by visualizing catheter tip vibration. The softer end of the straight-tip wire is then used to gently probe the valve in the area where leaflet separation was documented. Clockwise and counterclockwise catheter rotations are generally required to fall into the correct anteroposterior plane of the valve orifice. Once the valve is crossed, an attempt to fix the wire in the middle of the ventricular cavity is made, at which point the working view is switched to a right anterior oblique projection for further wire and catheter positioning (Figure 5–2, A-E). With the straight-tip wire fixed, the AL catheter is advanced into the left ventricle, avoiding advancement of the catheter tip and wire forcefully into the apex or other endocardial segments. Myocardial perforation can occur in this context. The catheter may be fixed in the mid-left ventricular chamber when removing the guide wire, allowing the broad secondary curve of the AL catheter tip to point back retrograde toward the aortic valve. A 0.035-inch J-tipped exchange wire can then be advanced through the AL catheter and easily draped in a broad curve across the anterior, apical, and inferior wall segments. A dual lumen pigtail catheter is then advanced into the left ventricle over the exchange wire, and simultaneous left ventricular and central aortic pressures are recorded to obtain a mean aortic valve gradient. Figure 5–2 A, Amplatz diagnostic catheter with straight-tip wire positioned in mid-left ventricular (LV) cavity. B, The Amplatz catheter tip directed retrograde toward the aortic valve while removing the straight-tip guidewire. C, Amplatz catheter positioned in mid-LV cavity after removing straight-tip guide-wire. D, 0.035-inch exchange wire being positioned in left ventricle and Amplatz catheter being withdrawn. E, 0.035-inch exchange wire draped in a broad curve across anterior and inferior LV wall segments before positioning the dual lumen pigtail catheter. Rapid ventricular pacing has been invaluable for stabilizing balloon position across the aortic valve during inflation. The marked decrement in forward stroke volume with pacing significantly reduces the likelihood of the balloon being ejected into the aorta. A bipolar pacing lead is positioned in the right ventricle via the right femoral venous sheath, and stable capture thresholds are confirmed. Some operators have reported success in attaching an external pacemaker directly to the left ventricular wire and skin using alligator clips to achieve rapid pacing without the need for a transvenous pacemaker. A brief pacing run is carried out at 180 to 220 beats/min to ensure consistent 1:1 capture without intermittent loss of capture or 2:1 exit block. A drop in systolic blood pressure to less than 50 mmHg also needs to be confirmed with this rapid pacing sequence (Figure 5–3). In patients with severe left ventricular systolic dysfunction, pacing at add 180 beats/min is often adequate to achieve significant drop in blood pressure. Test runs should be kept to a minimum to avoid provoking a transient decrease in LVEF and more prolonged hypotension. These high pacing rate commands can be given only by pacing from the atrial (not ventricular) connection terminal on the pacing box. A lower rate limit of 60 beats/min is programmed for backup pacing if necessary. The authors use an Amplatz Extra Stiff 0.035-inch exchange wire for the balloon valvuloplasty. Before exchanging this wire through the double-lumen pigtail catheter (or other diagnostic catheter) in the left ventricle, a broad loop is shaped in the flexible end either using a dull instrument or finger tips (Figure 5–4). Broader loops should be made for larger left ventricles. The curve is generally begun just proximal to the stiffer shaft transition into the more flexible segment, thereby avoiding a potential acute angled buckle in the wire capable of perforating the ventricle. In a right anterior oblique fluoroscopic projection, the wire is advanced into the ventricle and with the aid of the indwelling pigtail catheter, can be easily draped across the anterior, apical, and inferior wall segments. It is important to preserve this distal wire curve and left ventricular relationship during balloon inflation to avoid left ventricular perforations, which can occur during subsequent balloon inflations if the distal wire tip or sharply angled bend is pointed into the apex or inferior wall (Figure 5–5, A and B). Figure 5–5 A, Correct wire position during balloon inflation. B, Example of incorrect wire position with balloon inflation that can result in stiff wire “sawing” through left ventricular apex. Balloon sizing is generally more aggressive in stand-alone BAV than when predilation is carried out for TAVR. The authors use 4-cm noncompliant aortic balloons (e.g., Z-Med and Z-Med II [B. Braun Interventional Systems, Bethlehem, Pa.]) and generally begin with a balloon diameter-to-annulus ratio of 1:1 in stand-alone cases and 1 to 2 mm less when predilating for TAVR. The Maxi LD balloon (Cordis Corporation, Bridgewater, N.J.) is another popular choice; it is available in 4- or 6-cm lengths with a rated burst pressure of 5 to 6 atm and can be placed through smaller sheaths (8F to 12F) than the Z-Med balloons. More compliant balloons (e.g., Tyshak II [B. Braun Interventional Systems, Bethlehem, Pa.]) have the advantage of being lower in profile, and thus access sheaths ranging from 8F to 10F can be used. The more compliant balloons have less predictable inflation diameters, requiring the operator to use more conservative balloon diameters. In addition, rated burst pressures are just 2 atm or less. With these cylinder-shaped aortic balloons, antegrade migration can occur into the ascending aorta, even with rapid ventricular pacing. It is therefore important to choose a balloon diameter at least 1 to 2 millimeters less than the measured sinotubular junction diameter. Contrast used for balloon inflation is diluted to 1:8 or 1:10 to minimize viscosity and thus inflation/deflation times. The authors use a 60-cc handheld, disposable plastic syringe to inject the dilute contrast. Most balloons are filled using 20 to 30 cc of the dilute contrast. A 10-cc side syringe can be connected to a three-way stopcock on the balloon injection port to achieve more rapid filling or augment inflation volume. After positioning the uninflated balloon markers across the valve, rapid ventricular pacing is initiated. After confirming 1:1 capture and a systolic arterial blood pressure less than 50 mmHg, the balloon is inflated as rapidly as possible by the second operator or technician. The first operator holds the balloon catheter shaft, making adjustments under fluoroscopy to maintain a stable balloon position centered across the valve (Figure 5–6). The balloon is fully inflated for 2 to 3 seconds before deflation. Arterial pressure is monitored from the femoral arterial sheath side-arm. After balloon inflation, the contrast is aspirated as rapidly as possible, and the balloon pulled promptly back into the aorta. The pacer is not turned off until most of the contrast is removed from the balloon. Generally no more than 2 to 3 inflations are performed with each balloon size in stand-alone BAV. Subsequent inflations are not carried out unless the arterial blood pressure has completely returned to baseline. If necessary, we use 100 to 200 μ of phenylephrine in IV boluses to maintain systolic blood pressures greater than 100 mmHg. If there is difficulty squarely landing the inflated balloon on the valve, more rapid pacing rates may be beneficial, providing 1:1 capture is preserved. Initial subjective hemodynamic assessment often yields an obvious reduction in left ventricular systolic pressure and increase in aortic pressure (see Figure 5–7, A and B for pre-BAV and post-BAV gradients). In addition, the arterial waveform upslope is less delayed, demonstrating a more abrupt acceleration to peak systolic pressure. The attempt is to achieve a 50% reduction in the mean transvalvular gradient. Confirmation of a successful result requires repeating cardiac outputs to determine the AVA. Optimally, the intraprocedural valve area should increase by 40% to 50% or better. Figure 5–7 A, Pre-BAV simultaneous aortic and left ventricular pressure recordings, B, Post-BAV simultaneous aortic and left ventricular pressure recordings. A significant drop in the aortic diastolic blood pressure, especially if accompanied by an increase in left ventricular end-diastolic pressure, is strongly suggestive of acute, severe aortic insufficiency (AI) (Figure 5–8, A and B). Under severe circumstances, the diastolic aortic–to–end-diastolic left ventricular gradient may be less than 10 mmHg. Severe insufficiency is poorly tolerated in these patients and can rapidly progress to cardiogenic shock. At MHI, aortography is not routinely performed after BAV; when the patient is hemodynamically unstable, emergent transthoracic echocardiography (TTE) is performed. Figure 5–8 A, Baseline simultaneous ascending aortic and left ventricular pressure curves measured with a dual lumen pigtail catheter. Note severe aortic valve gradient before balloon valvuloplasty. B, Patient developed hemodynamic compromise after a single balloon inflation. Repeat simultaneous ascending aortic and left ventricular pressure measurements demonstrated significant reduction in the systolic gradient across the aortic valve; however, left ventricular end diastolic and aortic diastolic pressures are equal, characteristic of newly developed severe AI. The most relevant procedural complications include intraprocedural death, stroke, and vascular complications. Intraprocedural mortality has remained stable over the past decade and is surprisingly low with improvement in BAV technique. Multiple series document mortalities of 1% to 3%.9,17,19,26–28 Age or Society of Thoracic Surgery (STS) score does not appear to have a significant impact on this statistic.29 Autopsy-confirmed series documenting the precise causes are not available. In general, the catastrophic events leading to abrupt hemodynamic collapse include aortic root and annular dissection, valve leaflet avulsion with wide-open AI, left ventricular perforation, severe left ventricular dysfunction, and pulmonary hemorrhage as a complication of right heart catheterization. Strokes are consistently reported to occur in 1% to 2% of patients, and again the precise embolic origin has not been defined.17,26–28 Carotid protection devices should be of benefit to the extent that the embolic source would originate from the ascending and arch aorta or the aortic valve during the procedure. Severe AI is poorly tolerated in these patients with severe left ventricular hypertrophy and occurs consistently in 1% to 2% of BAVs.9,17,26,27 Severe AI can often be temporarily managed with rapid pacing to limit diastolic regurgitation. In addition, severe AI sometimes improves over several hours, likely in part from annular recoil. Vascular complications are considerably more variable, in part related to a previous lack of uniform definitions and poorly documented events in retrospective series. Vascular access site complications are now, however, clearly defined by the Valve Academic Research Consortium and divided into two broad categories: 1) major vascular complications and 2) minor vascular complications.29 Serious vascular complications include: those leading to death, those needing blood transfusions greater than 4 units, those requiring unplanned surgical repair, or those resulting in irreversible end-organ damage. Historically, complications requiring surgical intervention have occurred in at least 5% of BAV cases.8,26 Vascular access complications requiring unplanned surgical repair have been significantly reduced with closure devices, occurring in fewer than 1% in one series of patients undergoing BAV.25 1. Acute left ventricular systolic failure (especially in patients with baseline LVEF < 30%) 2. Left ventricular perforation with tamponade 3. Aortic valve annulus dissection or ruptured proximal aorta (with or without tamponade) 4. Valve leaflet avulsion and severe AI 5. Acute severe mitral insufficiency (mitral apparatus disruption secondary to balloon trauma or guidewire entrapment) 6. Brisk bleeding from the access site into the retroperitoneum or into an expanding hematoma 7. Vagally mediated hypotension 8. Bradyarrhythmia secondary to balloon-induced atrioventricular conduction injury 9. Sustained ventricular tachyarrhythmia secondary to guidewire position, after rapid ventricular pacing or prolonged ischemia The incidence of complete heart block requiring permanent pacemaker implantation is very low, much less than in recent TAVR series and generally in fewer than 1.5% of BAV procedures. Of 76 consecutive patients without a permanent pacemaker who underwent BAV at MHI, none required a permanent pacemaker in spite of the tendency to use large balloons and frequent dilations.30 The mean maximal balloon diameter in the series was 23.6 ± 0.1 mm, the maximal balloon/left ventricular outflow tract (LVOT) diameter ratio was 1.2 ± 0.1, and the mean number of balloon inflations was 3.9 ± 2.3. Five of the 76 consecutive patients developed new atrioventricular conduction defects (Table 5–3).30 New atrioventricular conduction defects were associated with a greater number of balloon inflations but not maximal balloon size or balloon/LVOT ratio in this small series. TABLE 5–3 New AV Conduction Abnormalities After BAV at Minneapolis Heart Institute∗ ∗Consecutive series of patients without permanent pacemakers. Pedersen W, Dang M, Krueger D, et al: Intra and post procedural outcomes in severe aortic stenosis patients with left ventricular ejection fractions <20% undergoing balloon valvuloplasty, Abstract. Catheter Cardiovasc Interv 2011;77(Suppl):S136-S137.
Aortic and Pulmonic Balloon Valvuloplasty
5.1 Balloon Aortic Valvuloplasty
Background
Valve Histopathology and Balloon Aortic Valvuloplasty Mechanism of Action
Patient Selection
Technique
Retrograde Approach
Patient Preparation and Access
Intraprocedural Diagnostic Evaluation
Crossing the Aortic Valve and Guidewire Positioning
Rapid Ventricular Pacing
Balloon Selection, Positioning, and Inflation
Intraprocedural Assessment and Goals
Intraprocedural Complications and Management
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Aortic and Pulmonic Balloon Valvuloplasty