FIGURE 15.1. Prosthetic grafts used for open surgical repair of aneurysms. Grafts may be made of knitted polyester fabric (shown here), woven fabric, or polytetrafluroethylene (ePTFE). (Image provided by MAQUET Medical Systems, USA.)
Juxtarenal aneurysms have no intervening segment of normal aorta distal to the renal arteries. With this type of aneurysm, endovascular repair is possible with specialized techniques or fenestrated grafts. Commercial fenestrated grafts became available in the in the United States in 2012, with the initial FDA approval for the treatment of AAAs with short infrarenal aortic necks (4 to 15 mm). The proximal segments of these custom-fabricated endografts have holes (fenestrations) so that stents can be placed into the renal arteries, thus providing secure proximal fixation.18 Endovascular repair also makes use of specialized techniques, including the use of endostapling (where the proximal graft is anchored to a short aortic neck), snorkeling, or the use of endografts that do not rely solely on proximal stent fixation. Open surgical repair is more common for the treatment of juxtarenal aneurysms, but suprarenal or supraceliac aortic clamping is required, increasing the risk of visceral ischemia during the procedure.
Suprarenal aneurysms involve the aorta above the renal arteries, and the renal arteries arise from an aneurysmal segment. Suprarenal aneurysms require concomitant renal or visceral artery reconstruction when open surgical repair is performed. Use of fenestrated or branched endografts for suprarenal aneurysms is an option in some cases, though still experimental in the United States in 2014.
Thoracic aortic aneurysms (TAA) cannot be evaluated by ultrasound due to imaging limitations from the air in the chest (ultrasound is not transmitted through the lungs). Thoracoabdominal aortic aneurysms (TAAA) can involve both the thoracic and abdominal segments of the aorta.19 Repair of a TAAA requires exposure of the aorta above and below the diaphragm. TAAAs are functionally classified considering the nature of surgical repair required.20 Suprarenal aortic aneurysms that extend proximally to include the celiac artery origin require aortic clamping and the proximal anastomosis be performed in the chest. This is a Type IV TAAA. Though the thoracic aorta cannot be visualized with ultrasound imaging, the possibility of a TAAA should be considered if the proximal extent of aneurysmal dilatation cannot be defined on an abdominal ultrasound examination. CT or MR angiography is needed for complete evaluation of any TAAA or suprarenal aneurysm.
Pathogenesis
Aneurysms can also be described by their morphology. The most common shape is fusiform—widest in the middle and tapering proximally and distally (Fig. 15.2). This descriptor is derived from the Latin fusus, meaning “spindle.” A more focal dilation is described as a saccular aneurysm. A saccular aneurysm resembles a small sac or outpouching. This term is derived from sacculus, the Latin word for small pouch or sac.
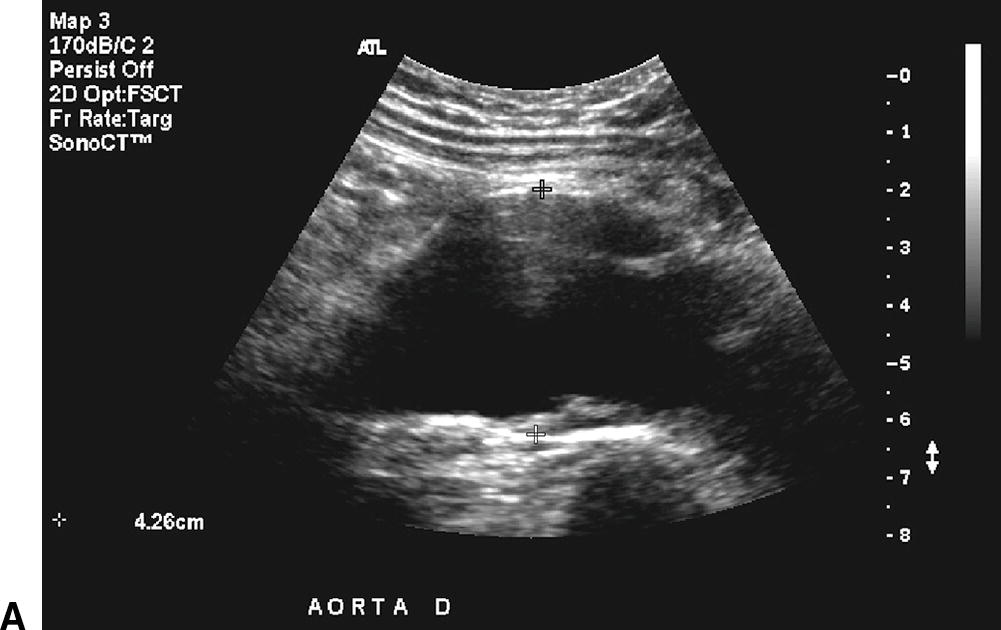
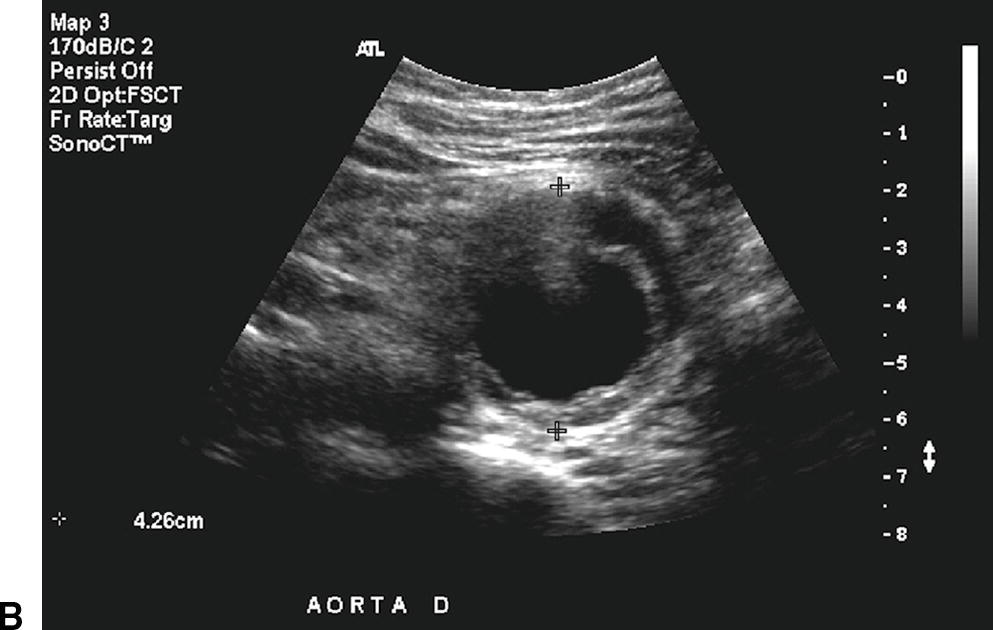
A,Longitudinal B-mode image of a fusiform aneurysm of the infrarenal aorta. The patient’s head is to the left of the image. Thrombus is visible in the aortic lumen, and the anterior-posterior (AP) diameter is 4.26 cm. B,Transverse B-mode image of the same abdominal aortic aneurysm at the site of maximum diameter (4.26 cm AP).
The most common cause of aortic and iliac artery aneurysms is a chronic degenerative change in the arterial wall leading to a focal arterial dilation. Though patients with aneurysms share risk factors with patients who have atherosclerosis,21 the term “atherosclerotic aneurysm” is no longer used, as the pathophysiology of AAA is distinct from atherosclerosis. The pathogenesis of an AAA is based on four broad cellular and molecular processes that include proteolytic degradation of the aortic wall, inflammation and immune responses, biomechanical wall stress, and molecular genetics.22 Multiple factors play a role in localized hemodynamic stress, and genetic predisposition attracts inflammatory cells (monocytes and lymphocytes) to the area of the infrarenal aorta.
Pseudoaneurysms, or “false” aneurysms, are the result of disruption of the arterial wall from injury or infection, or breakdown of an arterial anastomosis, such as the anastomosis of a prosthetic graft to a native artery (Fig. 15.3). Pseudoaneurysms tend to be saccular in appearance. Aneurysms occurring at, or adjacent to, the anastomosis of a graft (“juxta-anastomotic” or “para-anastomotic” aneurysms) are often pseudoaneurysms from disruption of the anastomosis, but they can also represent late aneurysmal degeneration of the remaining native vessel.23,24 Juxta-anastomotic aneurysms are rare enough that routine, regular surveillance is not recommended after uncomplicated open aortic procedures, at least not for several years after the initial operation.25 Pseudoaneurysm formation at one or more anastomotic sites can be a late presentation of a prosthetic graft infection.23,26–31
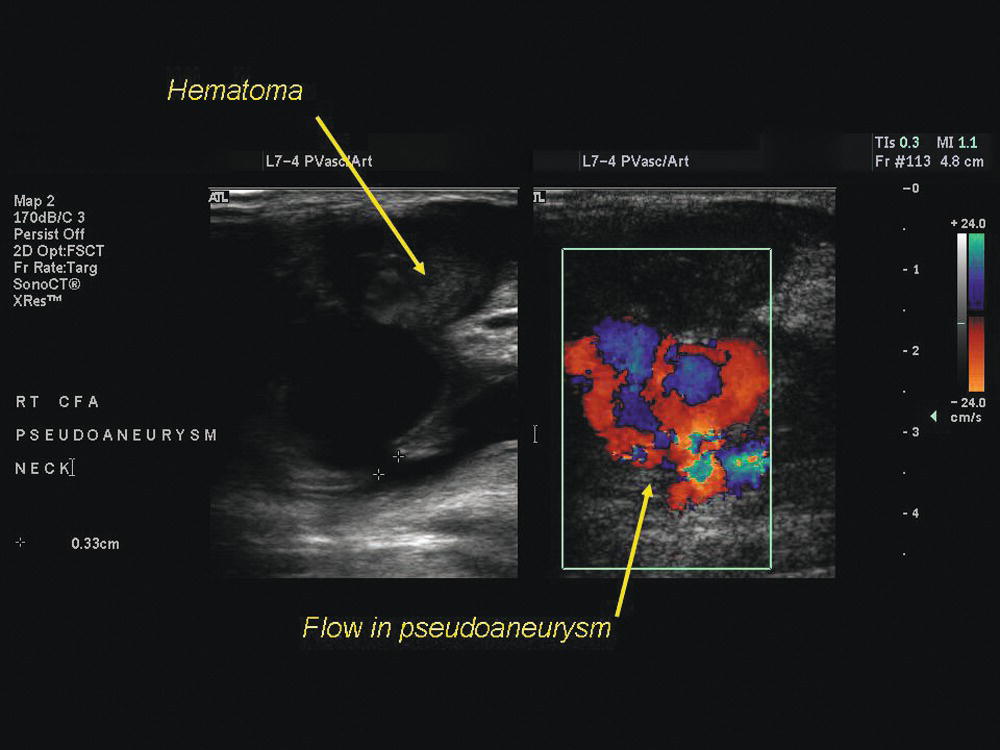
FIGURE 15.3. Pseudoaneurysm (false aneurysm) of the common femoral artery, a complication of arterial catheterization. There is an area of flow within the hematoma overlying the puncture site which communicates with the artery through a narrow “neck.”
Infection is an unusual cause for primary aneurysm formation, although the aorta, peripheral arteries, cerebral arteries, and visceral arteries (in descending order of frequency) can be affected by bacterial arteritis and false aneurysm formation. The most common causal pathogens are Staphylococcus and Streptococcus species. Imaging features of infected aneurysms include findings of a lobulated vascular mass, an indistinct irregular arterial wall, edema, or a perianeurysmal soft tissue mass. Perianeurysmal gas, aneurysm thrombosis, aneurysmal wall calcification, and disrupted arterial calcification have also been described with infected aneurysms, though these findings are less common.32
Threshold for AAA Treatment
Multicenter trials have found that there is no benefit for early surgical repair of small fusiform infrarenal AAAs (the most common type), compared to careful surveillance with serial ultrasound examinations.33,34 Small aneurysms can be safely observed.35 Based on a review of published data, the estimated average annual rate of rupture or acute expansion is almost nil for an AAA less than 4.0 cm in diameter.36 In a meta-analysis of 15,471 patients, small AAAs grew an average of 1.3 to 1.9 mm/year for 3.0 to 3.5 cm AAAs, 2.4 to 3.0 mm/year for 4.0 to 4.5 cm, and 3.6 mm/year for AAAs larger than 5.0 cm. Rupture rates of small aneurysms were found to be very low in this meta-analysis. The growth rates and rupture rates were found to be significantly higher in women than men.36
The Society for Vascular Surgery (SVS) guidelines for management of small aneurysms suggest serial ultrasound imaging at intervals that depend on aortic diameter.2 This strategy appears to be safe for men until the AAA reaches 5.5 cm in diameter, as the expected annual rupture rate is less than the risk of perioperative mortality with surgical repair observed in clinical trials.16,37–39 Repair is generally recommended if the expansion rate is greater than 0.5 cm in 6 months or if symptoms develop.40 Recommendations have been made to consider repair of AAAs at 5.0 cm in diameter for women, as rupture is more common in women with aneurysms, and female gender appears to be a risk factor for adverse outcomes.41–43
Because perioperative mortality with EVAR is less than with open repair, it has been suggested that EVAR could be considered at a smaller AAA size threshold. Two recently completed randomized controlled trials evaluated the efficacy of surveillance versus early EVAR for small AAAs. These trials found that mortality and rupture rates for AAAs less than 5.5 cm were low, and no clear advantage was shown with either an early or delayed EVAR strategy. Hence, the authors of both studies concluded that surveillance of small AAAs is safe if careful surveillance is possible.44–46
Screening and Ultrasound Diagnosis of AAA
Since AAAs are typically asymptomatic until rupture, screening for AAA is important. This allows AAA treatment in an elective setting. Physical examination is too insensitive to be relied upon for initial diagnosis, especially if the aneurysm is not large or if the patient is heavy,47 but over 90% of aortic aneurysms are infrarenal in location where ultrasound imaging is practical.
AAA screening has a demonstrated benefit. A large United Kingdom trial, the Multicentre Aneurysm Screening Study (MASS), was a randomized, controlled trial of over 65,000 population-based men 65 to 74 years of age who smoked at least 100 cigarettes in their lifetimes. One-half of the men were invited for AAA screening, while the control group was followed for clinical events but not invited for screening. The results of this multiyear study demonstrated the ability of screening to significantly reduce aneurysm-related deaths as well as reduce all-cause mortality.48–50 Recognizing the significance of this, Medicare has covered an aortic screening ultrasound (for new Medicare beneficiaries at risk) since 2007. Current recommendations call for a one-time ultrasound screening to be offered to men aged 65 to 75 years who have smoked 100 or more cigarettes in their lifetime or to men and women 50 years of age or older with a family history of AAA.14 In utilizing these criteria for screening a population at risk for AAA, a prevalence of 5.1% was detected in a contemporary series, which is similar to multiple other studies where the prevalence ranged from 3.9% to 7.2%.51
Aortic diameter measurements are used to diagnose an ectatic or aneurysmal aorta, stratify for risk of rupture, and guide the need for long-term follow-up. The normal diameter of the infrarenal aorta is generally less than 2 cm in transverse dimension. The aortic diameter is larger in men, and it increases with body size and age.52,53 An aneurysm is diagnosed when the abdominal aortic diameter is 1.5 times the normal value or when the aorta is 3 cm or greater in diameter. The aorta is deemed “ectatic” if the aorta measures 2 to 3 cm in diameter (greater than normal aortic size but less than 1.5 times the diameter of the undiseased adjacent aorta). Ultrasound follow-up is recommended for patients with aortic diameters of 2.6 to 2.9 cm at 5-year intervals, 3.0 to 3.4 cm at 3-year intervals, and for those with aortas of 3.5 to 4.4 cm, annual follow-up is recommended. Patients with larger AAAs of 4.5 to 5.4 cm in diameter should be followed at 6-month intervals.2
Ultrasonography is recommended for both initial screening and the diagnosis of AAA but is relatively imprecise in measuring aneurysm size. The Intersocietal Accreditation Commission Standards and Guidelines for Vascular Testing state that aneurysms should be measured at the widest point of the aorta from outer wall to outer wall (Fig. 15.4). The outer wall to outer wall measurement was found to have less intraobserver variability and has been suggested as the method for measuring AAA size in the large MASS trial.54 However, a more recent study has questioned the utility of outer to outer wall measurements and published data that interobserver reliability is greater with the inner wall to inner wall measurement.55
Ultrasound imaging may be limited in patients with large body habitus, overlying bowel gas, or those who are unable to cooperate with the examination. In these instances, an abdominal CT scan is more appropriate for making the initial diagnosis of AAA. CT is more reproducible for diameter measurement than ultrasound, and CT is the primary modality for surgical planning. However, in almost all other cases, ultrasound is the preferred modality for initial AAA diagnosis and follow-up.
Given the utility of ultrasonography in screening for AAAs, point-of-care examinations are being used more commonly, especially in underserved and rural areas where primary care clinics are performing AAA screening. The increasing availability of compact, low-cost, ultrasound systems is making point-of-care studies more commonplace, leading experts to believe that ultrasonography will be a basic skill in routine medical practice in the near future (see Chapter 27).56 The use of contrast-enhanced ultrasonography and 3D ultrasound have found some benefits in follow-up after EVAR, to detect endoleak or to find vascular branches (see Chapter 16), but these advanced applications are not particularly useful in the initial diagnosis of AAA.57
Patients presenting with a ruptured AAA need rapid evaluation and treatment. A focused ultrasound study for the presence of an AAA is part of the assessment of an elderly person with hypotension and abdominal pain.58,59 The diagnosis of AAA can be confirmed in a matter of minutes with a limited examination in the emergency department.60 If the clinical presentation suggests AAA rupture, there is no need to spend any additional time imaging to confirm the presence of retroperitoneal hematoma or hemoperitoneum, and transfer to the operating room for immediate treatment is indicated. However, a CT scan may be performed first if the patient is stable to guide planning of a possible endovascular repair.
PERIPHERAL ARTERY ANEURYSMS AND PSEUDOANEURYSMS
The utility of ultrasound imaging for the diagnosis and evaluation of peripheral artery aneurysms was established early.13 The low cost and low risk of ultrasound imaging make it the initial imaging modality of choice for peripheral artery aneurysm evaluations.
Popliteal Artery Aneurysms
The popliteal artery is the most common site for peripheral artery aneurysms, accounting for 70% of all peripheral aneurysms, though the overall incidence is quite low. Arterial diameters are normally larger in men than in women, but normal popliteal artery diameters are typically less than 1.0 cm.61,62 A popliteal artery is generally considered aneurysmal when the diameter is 1.5 cm, and repair is commonly recommended at 2 cm. Popliteal artery aneurysms occur much more frequently in men, are asymptomatic at the time of diagnosis in one-third, and bilateral in 50% of patients.63
Although no controlled trials exist regarding the management of popliteal artery aneurysms, a recent large multicenter registry demonstrated that there is equivalent efficacy between open and endovascular popliteal artery aneurysm repair.64 The rationale for repair is to prevent thrombosis or embolism, rather than rupture, as thromboembolic complications can lead to acute ischemia and limb loss, and rupture is rare.65 The reported risk of ischemic complications without surgical repair varies from 8% to 100% (mean 36%), depending on the selection of patients and duration of follow-up.63
Elective treatment is recommended for all symptomatic and most large asymptomatic popliteal artery aneurysms. Surgical reconstruction is the standard approach, but endovascular therapy with covered stents appears to be a therapeutic option, at least for some patients. Nonoperative observation with periodic ultrasound surveillance may be considered if the aneurysm measures less than 2.0 cm in diameter and contains no thrombus, or if the patient is at high surgical risk or has limited expected longevity.66
Femoral Artery Aneurysms
The common femoral artery (CFA) is the second most frequent site for peripheral artery aneurysms. True aneurysms of the CFA are fusiform and generally do not involve the deep femoral artery, though deep femoral artery aneurysms are seen in rare instances.67–72 The diameter of the CFA normally increases with age and is related to body size. CFA diameters are larger in males than in females.73 A CFA aneurysm is diagnosed when there is a focal dilatation of the vessel that is 1.5 to 2 times the diameter of the adjacent nondilated segment.
Symptomatic CFA aneurysms, those larger than 2.5 cm, and those associated with mural thrombus are treated surgically if patients have low operative risk and a reasonable life expectancy.66,71 However, nonoperative observation may be an option for some asymptomatic CFA aneurysms, as the risk for late complications appears to be less than that of popliteal artery aneurysms.66 Thorough evaluation for the extent of aneurysmal disease, the presence of concomitant occlusive disease, and the status of inflow and outflow vessels is needed for treatment planning.
Isolated superficial femoral artery (SFA) aneurysms are rare. They are most often found in elderly men and tend to involve the middle third of the artery. The most frequent clinical presentation is localized pain in association with a pulsatile mass. In contrast to popliteal artery aneurysms, SFA aneurysms more frequently present with rupture than with distal ischemia. SFA aneurysm repair is associated with favorable outcomes, with low reported rates of ischemia and limb loss.74
The detection of a peripheral artery aneurysm should prompt an evaluation for other aneurysms, including AAA. Finding a peripheral artery aneurysm is a strong predictor for the presence of an AAA, though the converse does not hold. Coexistent AAAs have been reported in 85% of patients with femoral aneurysms75 and in 62% of those with bilateral popliteal aneurysms,6 whereas femoral or popliteal aneurysms are present in only 3% to 14% of patients who have AAAs.76
Arteriomegaly
Arteriomegaly is the term used to describe diffuse arterial dilation, but this condition is also commonly associated with focal aneurysmal arterial segments.77–83 There is a risk of thrombosis and embolization with arteriomegaly, as flow is slow through the tortuous, ectatic, irregular vessels. Recognition of arteriomegaly in an arterial segment should prompt a thorough evaluation that includes assessment of the aorta as well as the femoral and popliteal arteries of both extremities. Diffuse enlargement of axial artery segments may also be seen in patients with chronic arteriovenous fistulae or arteriovenous malformations.
Pseudoaneurysm
False aneurysms or pseudoaneurysms of the femoral artery are far more common than true aneurysms in contemporary clinical practice, as the former may be the early or late result iatrogenic complications.
Late degeneration at the site of an arterial anastomosis can lead to pseudoaneurysm formation. This can be a consequence of suture line failure,84 degradation of graft material,85 or progressive degeneration of the tissue of the recipient vessel at the suture line.71,86–93 Anastomotic pseudoaneurysms are usually repaired with surgical revision by replacing the perianastomotic graft segment and reconstructing the arterial anastomosis. Late graft infection should be considered as a potential cause for a graft pseudoaneurysm.25,27,30,44,94,95 Perigraft fluid, local or systemic signs of inflammation, graft thrombosis, or the presence of multiple or recurrent pseudoaneurysms may suggest the diagnosis of infection. Extra-anatomic reconstruction or use of biologic grafts (autologous vein or arterial allograft) may be needed in such cases.
As discussed in Chapter 32, pseudoaneurysms as a complication of arterial catheterization are pulsatile hematomas that communicate with the artery through the residual defect in the arterial wall. Femoral pseudoaneurysms are well-recognized complications of arterial catheterization, occurring after 0.1% to 0.2% of diagnostic angiograms and after 3.5% to 5.5% of interventional procedures.66,71,96,97 Puncture-site pseudoaneurysms are associated with longer procedures, use of larger sheaths, systemic anticoagulation, and difficult arterial access. Catheter-related femoral pseudoaneurysms may be difficult to accurately detect with physical examination alone.
Both hematoma and pseudoaneurysm are in the differential diagnosis when ecchymosis and a lump in the groin are present after a femoral artery puncture. Duplex ultrasound, aided by the use of color flow imaging, is used to determine if there is flow outside the vessel, a finding that would confirm the presence of a pseudoaneurysm. In addition, ultrasound imaging can play a role in therapy for postcatheterization pseudoaneurysms, with either ultrasound-guided compression therapy97–102 or the more favored technique of ultrasound-guided thrombin injection.103–105
ULTRASOUND IMAGING OF ANEURYSMS: GENERAL CONSIDERATION
Ultrasound evaluation of an AAA should include abdominal aortic diameter measurement at the suprarenal, pararenal, and infrarenal locations. Common iliac artery diameters and, if indicated, graft anastomotic site diameters should also be included. Diameter measurements are made from a cross-sectional image of the vessel with the scan plane orthogonal to the long vessel axis. Two diameter measurements should be recorded. To avoid confusion about aneurysm size, aneurysm length measurements are generally not reported. A description of aneurysm morphology should be included, such a fusiform, saccular, or pseudoaneurysm.
An ultrasound evaluation of a peripheral artery aneurysm should include the maximum diameter of the artery, flow velocities, and patency of the outflow vessels.65 An ultrasound evaluation of a femoral pseudoaneurysm should include documenting the size of the pseudoaneurysm (both the active flow cavity and any thrombosed portion), and the flow velocities of the common femoral, superficial femoral, and deep femoral arteries. Also, the concomitant veins are evaluated for the presence of an arteriovenous fistula, venous thrombosis, or compression by the adjacent arterial pseudoaneurysm (see Chapter 32).
SCANNING TECHNIQUE: ABDOMINAL AORTA AND ILIAC ARTERY EVALUATION
Instrumentation
High-quality duplex ultrasound equipment facilitates evaluation of the abdominal aorta and iliac arteries. Transducers with frequencies of 2 to 5 MHz are typically selected for aortic studies to penetrate deep into the abdomen and pelvis. Linear or curved array transducers may be used to evaluate the abdominal aorta, but a low frequency phased array transducer may also be selected for its smaller footprint and good penetration.
Patient Positioning and Examination Preparation
Patients should be instructed to fast for 4 to 6 hours prior to duplex evaluations of the abdomen to reduce obscuring bowel gas and to improve the ease and quality of the study. Ideally, abdominal duplex scans should be scheduled in the morning. For afternoon appointments, a light breakfast may be permitted. Sips of water or small volumes of clear liquids may also be permitted, and all required medications should be taken. Examinations are performed without fasting if the patient needs to eat because of a medical condition or when medications need to be taken with food.
The patient is positioned supine on a gurney or examination table with the abdomen exposed. A small pillow or towel roll under the knees aids patient comfort. Some patients may have difficulty lying flat for various reasons: orthopnea from lung or cardiac conditions, neck and back pain from spinal or joint disease, or other problems. Patients may be more comfortable if the head of the bed is elevated approximately 10 degrees or by using reverse Trendelenburg position.
Imaging the abdominal vasculature can be challenging. Allow at least 60 minutes for an aortic evaluation. This provides sufficient time to attend to setup of the scan room, gather patient information, prepare the patient, perform the examination, record images, and generate the report. The technologist should scan using an ergonomically comfortable position, and the patient should be positioned on the gurney as close as possible to the technologist. This lessens the “reach” required and reduces the stress placed on the technologist’s wrist, elbow, shoulder, and back. Positioning the scanned surface below the level of the technologist’s waist allows for easier reach to all of the abdomen and better leverage. It is important for the technologist to keep their elbow as straight as possible, as this can lessen joint stress and reduce the likelihood of repetitive strain injuries. For larger patients, it may be necessary to lean into the patient and put more pressure on the abdomen to get the transducer closer to the aorta. A small step stool may be used by shorter technologists to gain the needed height over the patient’s abdomen.
Imaging Views and Protocols
The aortic evaluation is begun with transverse imaging, starting at the level of the of the xyphoid process. The visceral segment of the abdominal aorta should be identified at the level of the celiac and superior mesenteric artery origins. The transducer is moved from cephalad to caudal, with transverse imaging of the aorta from the celiac axis to the aortic bifurcation. The umbilicus is the surface anatomy landmark for the approximate location of the aortic bifurcation which is typically at the level of the second lumbar vertebra. Initial examinations should also include imaging of the common iliac, external iliac, and internal iliac arteries, bilaterally.
Transverse diameter measurements should be recorded at multiple levels, including the suprarenal, pararenal, and infrarenal aorta, and at the terminal aorta just proximal to the origins of the common iliac arteries. Two diameter measurements of the aorta are made at each location: the anterior-posterior (AP) and the lateral dimensions. The lateral measurements are usually less accurate due to the lower lateral resolution of B-mode ultrasound compared to its depth resolution (Fig. 15.4).
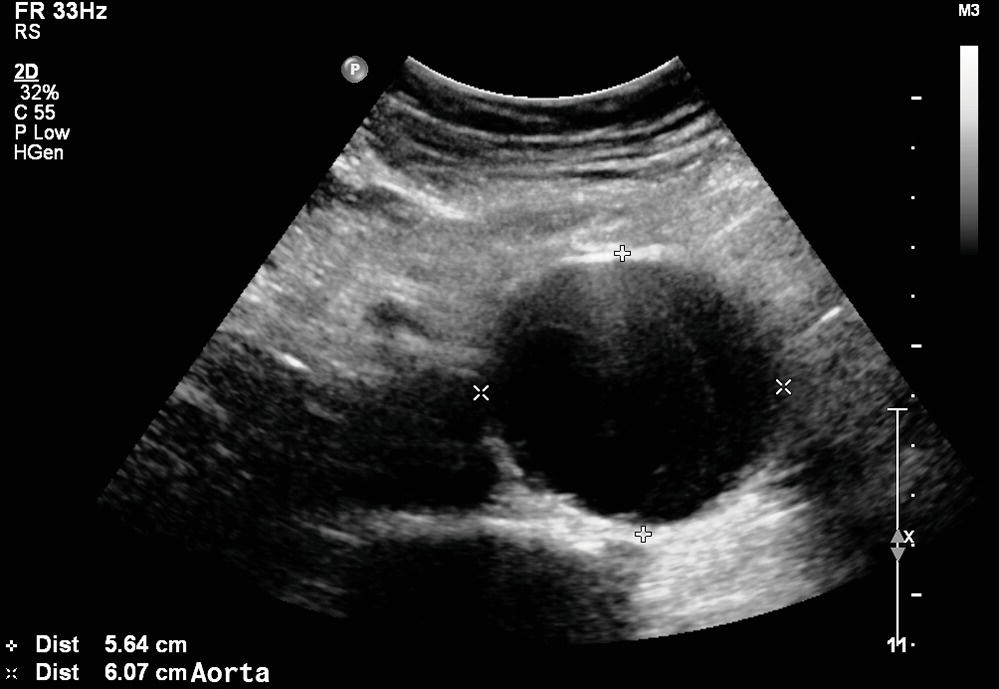
FIGURE 15.4. B-mode image of an abdominal aortic aneurysm in transverse view. The abdominal wall is the most superficial structure at the top of the image, and the vertebral column is deep to the aorta at the bottom of the image. Specular reflections from the deep and superficial walls of the aorta (+) are more distinct than those from the lateral walls (×). Therefore, the lateral resolution of ultrasound is less that the depth resolution, making accurate anterior-posterior (AP) diameter measurements easier to obtain.
The suprarenal aorta is generally not parallel to the skin surface. Because of the rigidity of the thoracic cage, the ability to compress with the probe is limited in the upper abdomen, and imaging with optimum alignment of the suprarenal aorta may be difficult. The aorta will appear on the monitor to be rising from a deeper to a more superficial position. Care should be taken to avoid off-axis measurements that overestimate the true diameter. Angle the transducer to obtain true axial diameter measurements. The aorta can also be imaged in a longitudinal (sagittal) plane (See Fig. 15.2A). A single AP measurement is obtained with the longitudinal view.
Color flow Doppler can help to identify the visceral and renal arteries (Fig. 15.5). In the transverse view, the right renal artery will often arise at a 10-o’clock orientation, and the left renal artery will arise at a 4-o’clock position. In many cases, the right renal artery will arise from the aorta more superiorly than the left renal artery. The pararenal aortic diameter can be obtained at either location.
Most AAAs originate below the level of the renal arteries.16 The infrarenal aorta is the most easily visualized aortic segment, owing to the shallower depth. In the presence of an AAA, the increased diameter makes the aorta easy to recognize. Mural thrombus is often identified (Figs. 15.6 and 15.7). The imaging plane is manipulated to be at right angles to the axis of the aorta to best measure the true diameter (Fig. 15.8). Aneurysmal aortas are often tortuous due to elongation of the aorta, and off-angle imaging will yield a more elliptical cross section. While the minor axis diameter of the elliptical section may be close to the true aortic diameter, the major axis measurement of the elliptical section may result in overestimation of the AAA size. The terminal aorta will often be elliptical in shape, with the lateral measurement greater than the AP measurement.
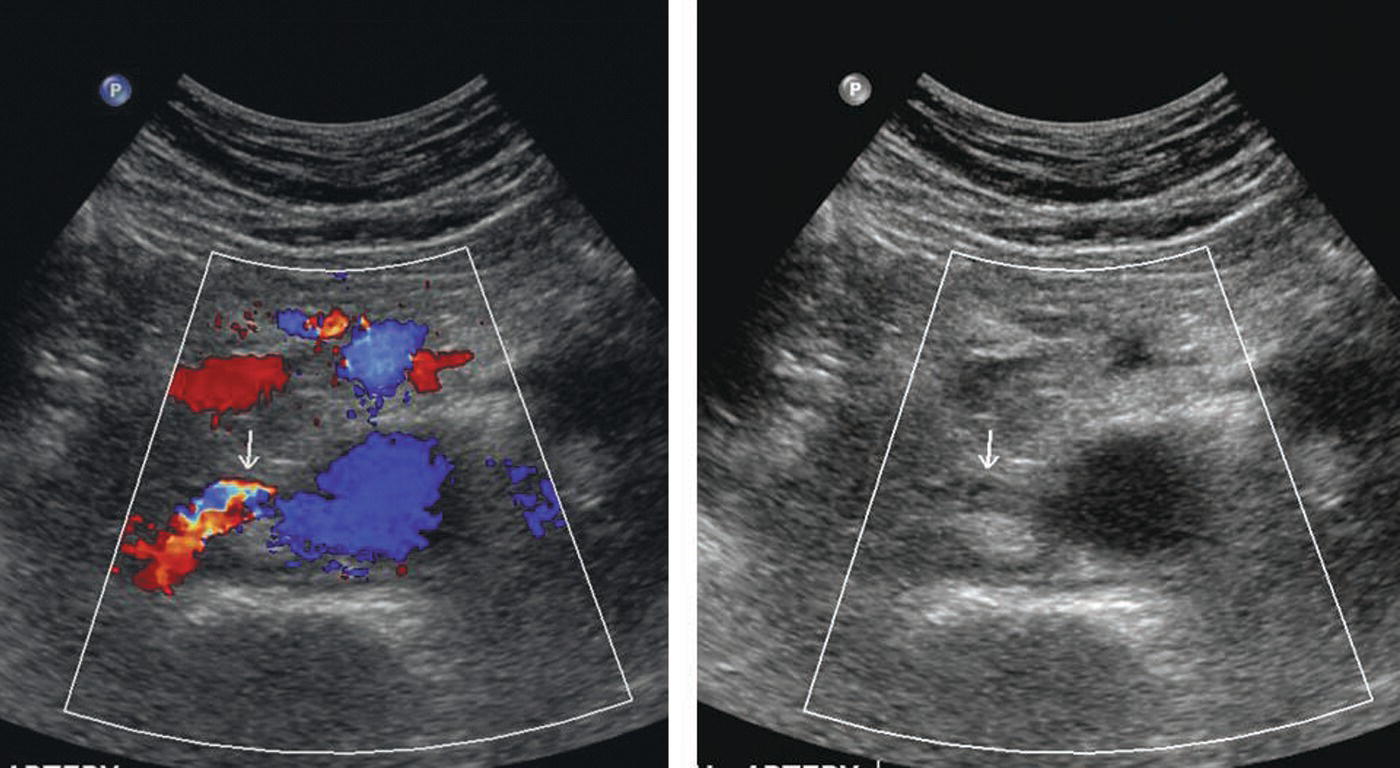
FIGURE 15.5. The use of color Doppler helps to quickly identify renal arteries. Transverse color Doppler image (left) and B-mode image alone (right) showing a right renal artery (arrows).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


