Chapter 16 Antiinflammatory Drugs
Corticosteroids
Pharmacodynamics
Cellular, Tissue, and Systemic Effects
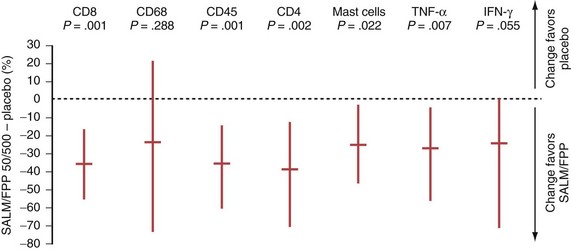
In COPD, however, even high-dose inhaled corticosteroids have little effect on airway inflammation, although they are effective when combined with a long-acting β2-agonist (LABA) (Figure 16-1).
Molecular Mechanisms
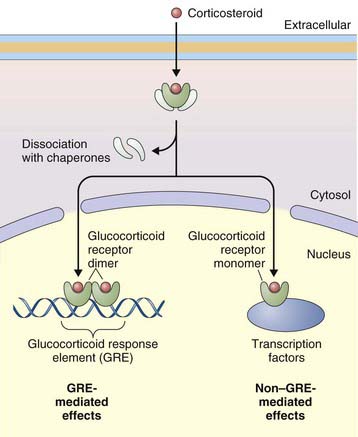
The primary effect of corticosteroids appears to be at the genetic level, activating transcription of antiinflammatory genes and repressing proinflammatory genes. They act primarily by binding to intracellular glucocorticoid receptors, which in turn regulate gene expression through glucocorticoid response elements (GREs) (Figure 16-2). Once inside the nucleus, glucocorticoid receptors dimerize and bind to GREs in the promoter regions of steroid-responsive genes, altering gene transcription and initiating a cascade of antiinflammatory effects downstream. Mediators affected by corticosteroids include cytokines, adhesion molecules, and chemokines. Nuclear glucocorticoid receptor monomers also interact with transcription factors such as nuclear factor (NF)-κB, to suppress expression of a number of proinflammatory genes. Corticosteroids can also decrease protein synthesis by decreasing messenger RNA (mRNA) stability.
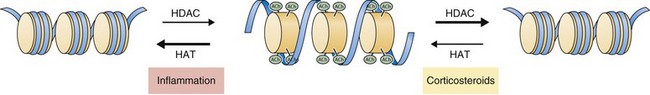
Recent work has focused on the role of corticosteroids in regulating gene expression through effects on histone acetylation and chromatin compaction. Inflammatory signals cause chromatin unwinding by histone acetyltransferase activity. Corticosteroids can directly inhibit histone acetyltransferases and recruit histone deacetylases (HDACs) to their site of action, seemingly independent of glucocorticoid receptor binding to GREs. Corticosteroids interact with specific HDACs (such as HDAC2) that target specific histone proteins (e.g., histone H4), regulating expression of particular regions of the genome. The net effect is to decrease histone acetylation, promoting chromatin compaction and downregulation of inflammatory gene expression (Figure 16-3).