Fig. 2.1
Thromboelastograph (TEG) parameters
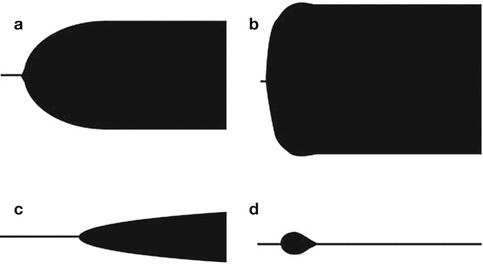
Fig. 2.2
Schematic presentation of various TEG tracings in trauma: (a) normal, (b) hypercoagulability, (c) hypocoagulability (thrombocytopenia/thrombocytopathy), and (d) primary hyperfibrinolysis
Reduced clot stability correlates with clinical bleeding conditions as demonstrated by Plotkin et al. [50] who, in patients with a penetrating trauma, reported TEG to be an accurate indicator of the blood product requirements. Furthermore, TEG is the gold standard for identifying hypercoagulability [51] and hyperfibrinolysis [29, 30, 52], the latter a significant cause of bleeding in patients with major trauma [29, 30, 52]. The use of VHA in trauma is now recommended by recent guidelines [53] and textbooks [54] and its use in the military field is extensive, and many level 1 trauma centers consider the use of VHA to monitor and guide hemostatic therapy as routine; a recent study of 1.974 trauma patients report that rapid TEG can replace the conventional coagulation tests in trauma patients [55].
2.5.2 Whole Blood Platelet Function Analyzers
Different assays evaluating the degree of platelet inhibition secondary to platelet inhibitors exist. Light transmission aggregometry (LTA), previously considered the “gold standard” for investigation of platelet aggregation [56], unfortunately relies on artificially manufactured platelet-rich plasma suspensions, which do not reflect in vivo conditions, and consequently platelet function assays performed on whole blood are today favored. Examples of whole blood platelet function assays are PFA100 (Siemens, Tarrytown, USA), VerifyNow (Accumetrics, San Diego, USA), Multiplate (Verum Diagnostica GmbH, Munich, Germany), and TEG PlateletMapping (Haemonetics Corp, Chicago, USA), which all have been reported to be able to identify clinically relevant platelet inhibition secondary to pharmacological platelet inhibitors.
2.6 Administration of Blood Products
2.6.1 Red Blood Cells
In response to hemorrhage, lowered hematocrit contributes to coagulopathy since erythrocytes promote marginalization of platelets so the platelet concentrations along the endothelium remain almost seven times that of the average blood concentration [57]. In addition, erythrocytes support thrombin generation through exposure of procoagulant membrane phospholipids [58], and they activate platelets by liberating ADP [59] emphasizing that in vivo thrombus formation is a multicellular event [44, 60]. Yet, the optimal hematocrit for platelet-vessel wall interactions remains unknown but it may be as high as 35 % [61].
2.6.2 Fresh Frozen Plasma
It remains controversial when and in what dose plasma should be transfused to massively bleeding trauma patients [62], and the optimal ratio of FFP to RBCs remains to be established although collectively the data indicate that a FFP/RBC ratio greater than 1:2 is associated with improved survival compared to one lower than 1:2 [63, 64]. This is further supported by a review and meta-analysis from 2010 reporting that in patients undergoing massive transfusion, high FFP to RBC ratios were associated with a significant reduction in the risk of death (odds ratio (OR) 0.38 (95 % CI 0.24–0.60)) and multiorgan failure (OR 0.40 (95 % CI 0.26–0.60)) [65] and a meta-analysis from 2012 reports of reduced mortality in trauma patients treated with the highest FFP or PLT to RBC ratios [66].
2.6.3 Platelets
Platelets are also pivotal for hemostasis [44, 66], and several retrospective studies report an association between thrombocytopenia and postoperative bleeding and mortality [67, 68]. Holcomb et al. [63] found that the highest survival was established in patients who received both a high PLT/RBC and a high FFP/RBC ratio. Inaba et al. recently reported from a retrospective study of massively transfused patients that as the apheresis platelet to RBC ratio increased, a stepwise improvement in survival was seen and a high apheresis PLT/RBC ratio was independently associated with improved survival [69]. This is in alignment with Brown et al. who reported that admission platelet count was inversely correlated with 24-h mortality and transfusion of RBCs and that a normal platelet count may be insufficient after severe trauma, suggesting these patients may benefit from a higher platelet transfusion threshold [70]. In a recent meta-analysis, a high PLT/RBC ratio in massively bleeding trauma patients was reported to reduce mortality [66].
2.6.4 Massive Transfusion Protocols and Ratios
A recent meta-analysis of retrospective observational studies evaluating the effect of FFP/RBC and/or PLT/RBC ratios and survival in massively bleeding trauma patients recently reported a significant survival benefit in patients receiving high FFP/RBC and PLT/RBC ratios [66]. A potential confounder of these results is survivorship bias relating to that those surviving long enough will receive FFP and PLT whereas those dying early will not, as reported by Snyder et al. [71]. Survivorship bias, however, does not explain the improved survival in studies concerning the introduction of transfusion packages where both FFP and PLT are immediately available, i.e., where pre-thawed FFP are available. Cotton et al. [72] implemented a trauma exsanguination protocol (TEP) involving 10 RBC, 4 FFP, and 2 apheresis PLT for trauma patients and used it to evaluate 211 trauma patients of who 94 received TEP and 117 were historic controls. The TEP patients received more RBC (16 vs. 11), FFP (8 vs. 4), and PLT (2 vs. 1) intraoperatively than the controls and displayed lower 30-day mortality (51 % vs. 66 %). After controlling for age, sex, mechanism of injury, Trauma and Injury Severity Score (TRISS), and 24-h blood product usage, a 74 % reduction in the odds ratio of mortality was found among patients in the TEP group. In a later study involving additionally 53 patients, Cotton also [73] reported that not only was 30-day survival higher in the TEP group compared to the controls but the incidence of pneumonia, pulmonary failure, and abdominal compartment syndrome was lower in the TEP patients. Also, the incidence of sepsis or septic shock and multiorgan failure was lower in TEP patients. In alignment with this, Duchesne et al. reported that in trauma patients undergoing damage control laparotomy, introduction of damage control resuscitation encompassing early administration of plasma and platelets together with RBC was associated with improved 30-day survival (73.6 % vs. 54.8 %, p < 0.009) when compared to patients treated with conventional resuscitation efforts [74]. A multicenter randomized control study evaluating the effect of different blood product ratios on survival in massively bleeding trauma patients will commence in the USA this year, and hopefully this will result in evidence for how best to resuscitate these patients with blood products (http://www.uth.tmc.edu/cetir/PROPPR/index.html).
2.6.5 Fresh Whole Blood
With the implementation of fractionated blood components, the routine use of fresh whole blood (FWB) for resuscitation of bleeding patients was abandoned in the civilian setting. In the combat setting, however, FWB has been used in situations where fractionated blood products and especially platelets were not available. In a report of US military patients in Iraq and Afghanistan from January 2004 to October 2007, those with hemorrhagic shock, a resuscitation strategy that included FWB was associated with improved 30-day survival (95 % vs. 82 %, p = 0.002) [75], and an ongoing trial is currently addressing this issue (www.clingovtrial.com/NCT01227005). It should be noted that administration of any blood product carries potential risks for the patient including viral and bacterial transmission, hemolytic transfusion reactions, transfusion-related acute lung injury, and immunomodulation and consequently transfusion of blood products should be reserved to patients who actually needs this therapy [76].
2.7 Goal-Directed Hemostatic Resuscitation with Transfusion Packages Together with VHA
Goal-directed treatment with blood products and antifibrinolytic pharmacological agents based on the result of the whole blood viscoelastic hemostatic assays (VHA), together with the clinical presentation, was introduced for more than 25 years ago in patients undergoing liver transplantation and cardiac surgery, and there are validated algorithms for how coagulopathy is identified and treated in patients with ongoing bleeding, based on VHA [77]. More than 25 studies encompassing more than 4,500 patients have evaluated VHA vs. conventional coagulation assays on bleeding and transfusion requirements in patients undergoing cardiac, liver, vascular, or trauma surgery and in patients requiring massive transfusion. These studies demonstrate the superiority of VHA in predicting the need for blood transfusion, and the VHA-based algorithm reduces the transfusion requirements and the need for redo surgery in contrast to the treatment based on conventional coagulation assays [49], and this was further corroborated in a recent Cochrane review [78].
We found that the implementation of goal-directed hemostatic resuscitation of massively bleeding patients, including trauma, based on the VHA reduced mortality by approximately 30 % in patients requiring more than 10 RBC in the first 24 h [77], and recently we reported that early hemorrhagic death in trauma patients was reduced by more than 40 % when a combination of transfusion packages and goal-directed hemostatic resuscitation was employed [79]. The systematic use of VHA to monitor and guide transfusion therapy is furthermore endorsed by several recent international transfusion guidelines and teaching books [68, 69].
2.8 Hemostatic Agents
2.8.1 Antifibrinolytics
Hyperfibrinolysis contributes significantly to coagulopathy, and antifibrinolytic agents reduce the blood loss in patients with both normal and exaggerated fibrinolytic responses to surgery by preventing plasmin(ogen) from binding to fibrin and by preventing plasmin degradation of platelet glycoprotein Ib receptors [80]. In a placebo-controlled randomized study (CRASH-2) including 20,211 adult trauma patients, tranexamic acid as compared to placebo significantly decreased all-cause mortality from 16.0 to 14.5 %, p = 0.0035 [81]. We recommend monitoring of hemostasis with TEG to identify hyperfibrinolytic states in trauma patients and, consequently, targeted treatment with antifibrinolytic agents.
2.8.2 Recombinant Factor VIIa
Recombinant factor VIIa (rFVIIa) acts in pharmacological doses by enhancing thrombin generation on the activated platelets independent of factors VIII and IX and is currently approved for episodes of severe hemorrhage or perioperative management of bleeding in patients with congenital factor VII deficiency and hemophilia A or B with inhibitors [82]. Data from the CONTROL trial, a phase 3 randomized clinical trial evaluating the efficacy and safety of rFVIIa as an adjunct to direct hemostasis in major trauma, rFVIIa did not change mortality in patients with blunt (11.0 % (rFVIIa) vs. 10.7 % (placebo)) or penetrating (18.2 % (rFVIIa) vs. 13.2 % (placebo)) trauma [83]. In a recent review reporting on the rate of thromboembolic events in all published randomized placebo-controlled trials of rFVIIa use, Levi [84] reported that the rates of arterial thromboembolic events among all subjects were higher among those who received rFVIIa than among those who received placebo (5.5 % vs. 3.2 %, p = 0.003).
2.8.3 Fibrinogen Concentrate
Conversion of sufficient amounts of fibrinogen to fibrin is a prerequisite for clot formation, and reduction in the circulating level of fibrinogen due to consumption and/or dilution by resuscitation fluids induces coagulopathy [85]. It is, therefore, important to maintain an adequate fibrinogen level when continued bleeding is bridged by saline and/or colloid infusion or blood products (primarily RBC) without fibrinogen. Recent data indicate that coagulopathy induced by synthetic colloids such as HES may be reversed by the administration of fibrinogen concentrate [86]. A recent review only found four studies of poor quality assessing fibrinogen concentrate to bleeding surgical and trauma patients and concluded that randomized controlled trials of sufficient size and long-term follow-up need to be performed before such a practice can be recommended routinely [87]. The use of fibrinogen concentrate in patients with established hypofibrinogenemia, diagnosed by VHA (Functional Fibrinogen® or FibTEM®), in addition to a balanced administration of RBC, FFP, and platelets, may however contribute to faster achievement of a normal hemostasis in massively bleeding patients, and we recommend the use of fibrinogen concentrate according to TEG-guided algorithms in such patients.
2.8.4 Prothrombin Complex Concentrate
The four-factor PCC encompasses coagulation factors II, VII, IX, and X that all are essential for thrombin generation. Administration of PCC is indicated for the treatment of congenital coagulation disorders and to reverse orally administered anticoagulation by vitamin K antagonists [88], whereas experience with treatment of massive bleeding with PCC is lacking. Recently, Carvalho et al. reported that the administration of PCC to patients with massive bleedings had beneficial effect on hemostasis and this warrants further investigation in a randomized controlled setting [89].
2.8.5 Factor XIII
Factor XIII is important for clot firmness by binding to platelets through the GPIIb/GPIIIa receptor and by cross-linking fibrin and increasing the resistance of the formed clot against fibrinolysis [90]. Notably, patients with “unexplained” intraoperative coagulopathies and, hence, bleeding demonstrate significantly less FXIII per unit thrombin available both before, during, and after surgery [91]. An increased tendency to postoperative bleeding has been observed, even at factor XIII activities as high as 60 % [92]. The role of FXIII administration to bleeding trauma patients however remains to be investigated in randomized clinical trials.
2.9 Conclusion
Viscoelastic whole blood assays, such as TEG and ROTEM, are advantageous for identifying coagulopathy and guide ongoing transfusion therapy. From the result of these assays, the implementation of a hemostatic control resuscitation strategy to massively bleeding patients seems both reasonable and lifesaving although data from prospective randomized controlled trials are lacking. Until a definite proof from such trials is available, retrospective data support a shift in transfusion medicine in regard to early and aggressive administration of plasma and platelets.
References
1.
2.
4.
Dutton RP. Resuscitative strategies to maintain homeostasis during damage control surgery. Br J Surg. 2012;99 Suppl 1:21–8. doi:10.1002/bjs.7731.PubMedCrossRef
5.
American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208.CrossRef
6.
7.
Mittermayr M, Streif W, Haas T, Fries D, Velik-Salchner C, Klingler A, Oswald E, Bach C, Schnapka-Koepf M, Innerhofer P. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesth Analg. 2007;105:905–17.PubMedCrossRef
8.
9.
10.
Neal MD, Hoffman MK, Cuschieri J, Minei JP, Maier RV, Harbrecht BG, Billiar TR, Peitzman AB, Moore EE, Cohen MJ, Sperry JL. Crystalloid to packed red blood cell transfusion ratio in the massively transfused patient: when a little goes a long way. J Trauma Acute Care Surg. 2012;72:892–8.PubMed
11.
Duchesne JC, Guidry C, Hoffman JR, Park TS, Bock J, Lawson S, Meade P, McSwain Jr NE. Low-volume resuscitation for severe intraoperative hemorrhage: a step in the right direction. Am Surg. 2012;78:936–41.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


