The prevalence, intensity, safety, and efficacy of oral anticoagulation (OAC) in addition to dual antiplatelet therapy (DAPT) in “real-world” patients with atrial fibrillation (AF) undergoing percutaneous coronary intervention (PCI) have not yet been fully evaluated. In the Coronary REvascularization Demonstrating Outcome Study in Kyoto registry cohort-2, a total of 1,057 patients with AF (8.3%) were identified among 12,716 patients undergoing first PCI. Cumulative 5-year incidence of stroke was higher in patients with AF than in no-AF patients (12.8% vs 5.8%, p <0.0001). Although most patients with AF had CHADS 2 score ≥2 (75.2%), only 506 patients (47.9%) received OAC with warfarin at hospital discharge. Cumulative 5-year incidence of stroke in the OAC group was not different from that in the no-OAC group (13.8% vs 11.8%, p = 0.49). Time in therapeutic range (TTR) was only 52.6% with an international normalized ratio of 1.6 to 2.6, and only 154 of 409 patients (37.7%) with international normalized ratio data had TTR ≥65%. Cumulative 5-year incidence of stroke in patients with TTR ≥65% was markedly lower than that in patients with TTR <65% (6.9% vs 15.1%, p = 0.01). In a 4-month landmark analysis in the OAC group, there was a trend for higher cumulative incidences of stroke and major bleeding in the on-DAPT (n = 286) than in the off-DAPT (n = 173) groups (15.1% vs 6.7%, p = 0.052 and 14.7% vs 8.7%, p = 0.10, respectively). In conclusion, OAC was underused and its intensity was mostly suboptimal in real-world patients with AF undergoing PCI, which lead to inadequate stroke prevention. Long-term DAPT in patients receiving OAC did not reduce stroke incidence.
It has been reported that 5% to 10% of patients undergoing percutaneous coronary intervention (PCI) have concomitant atrial fibrillation (AF). Most of those patients have an indication for oral anticoagulation (OAC) to prevent stroke or systemic thromboembolism and also for antiplatelet therapy to prevent ischemic cardiac events, particularly stent thrombosis (ST). Drug-eluting stents (DES) has become widely used, and dual antiplatelet therapy (DAPT) with aspirin plus thienopyridine for ≥12 months is recommended after DES implantation. Thus, patients with AF undergoing PCI often have an indication for long-term use of OAC plus DAPT, although a great concern about bleeding complications has been raised for such a “triple” antithrombotic therapy. However, the prevalence and intensity as well as the safety and efficacy of OAC in combination with DAPT in “real-world” patients with AF undergoing PCI have not yet been fully evaluated. For patients receiving triple therapy in the real-world clinical practice, OAC could be less intensive because of a concern about bleeding complications. It is unknown, however, whether the less-intensive OAC in patients receiving concomitant DAPT is effective in preventing stroke. Also unknown is the effect of DAPT on long-term cardiovascular outcomes in patients receiving concomitant OAC. Consequently, we investigated the practice pattern and outcome regarding OAC and DAPT use in patients with AF in a large observational PCI database in Japan with 4 to 7 years of follow-up.
Methods
The Coronary REvascularization Demonstrating Outcome Study in Kyoto (CREDO-Kyoto) registry cohort-2 is a physician-initiated, non–company sponsored, multicenter registry enrolling consecutive patients undergoing first coronary revascularization among 26 centers in Japan from January 2005 to December 2007. The relevant review boards or ethics committees in all 26 participating centers ( Supplementary Appendix A ) approved the research protocol.
A total of 15,939 patients undergoing first coronary revascularization were enrolled in the registry. We excluded 99 patients who refused study participation, 2,782 who underwent coronary artery bypass grafting, and 342 who died during the index hospitalization. Thus, the present study’s population consisted of 12,716 patients undergoing first PCI who were alive at hospital discharge ( Figure 1 ).
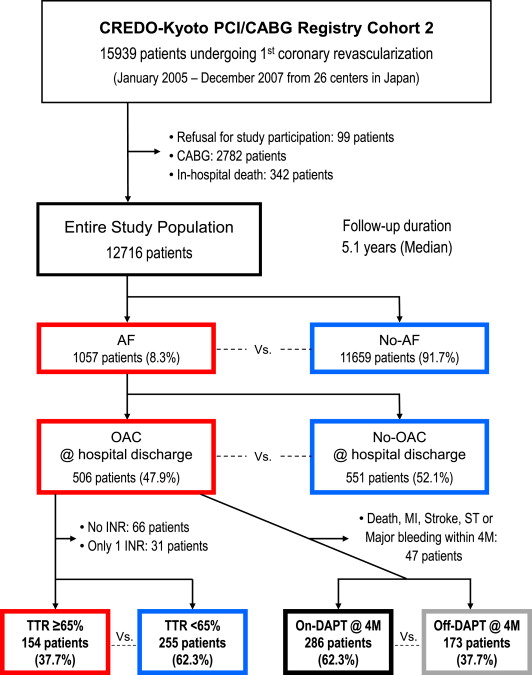
Recommended antiplatelet regimen for PCI stenting was aspirin (≥81 mg/day) indefinitely and thienopyridine (200-mg ticlopidine or 75-mg clopidogrel per day) for at least 3 months. Choices regarding duration of DAPT and administration of warfarin in patients with AF were left to the discretion of each attending physician. Persistent discontinuation of the antithrombotic drugs was defined as withdrawal lasting for at least 2 months. Withdrawal of DAPT was defined as persistent discontinuation of either aspirin or thienopyridine.
We defined patients with AF as those with a preexisting diagnosis of AF and those who developed new-onset AF during their index hospitalization. The primary outcome measure was stroke including both ischemic and hemorrhagic strokes. Stroke was defined as an acute onset of a focal neurologic deficit of presumed vascular origin requiring hospitalization with symptoms lasting >24 hours or resulting in death. The types of strokes were distinguished by imaging studies to be either hemorrhagic or ischemic. Cerebral bleeding that occurred secondary to ischemic stroke was not regarded as hemorrhagic stroke.
The secondary outcome measures were all-cause death, myocardial infarction (MI), ST, and major bleeding. ST was defined as Academic Research Consortium definite ST. Major bleeding was defined as moderate or severe bleeding by Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries classification.
Demographic, angiographic, and procedural data were collected from hospital charts or hospital databases according to prespecified definitions by experienced clinical research coordinators from the study management center (Research Institute for Production Development, Kyoto, Japan; Supplementary Appendix B ). Follow-up data were obtained from hospital charts or by contacting patients or physicians in charge. All the primary and secondary end points were adjudicated by the independent clinical events committee ( Supplementary Appendix C ).
Data for international normalized ratio (INR) during follow-up in patients with AF receiving OAC were collected from the hospital charts of the centers where the index PCI was performed. Time in therapeutic range (TTR) in the OAC group was calculated by the Rosendaal method, according to a therapeutic INR range of 1.6 to 2.6, which is recommended for elderly (≥70 years) patients in the Japanese guidelines. Because the stroke event may affect the intensity of subsequent OAC, TTR in patients with such an event during follow-up was calculated only using INR data before or at the time of the stroke.
Data are presented as values and percentages, mean value ± SD, or median with first quartile to third quartile (Q1 to Q3). Categorical variables were compared with the chi-square test or the Fisher’s exact test. Continuous variables were compared using the Student t test or Wilcoxon rank sum test based on their distributions. Cumulative incidence was estimated by the Kaplan-Meier method, and differences were assessed with the log-rank test.
We used the Cox proportional hazards model to adjust for the differences in baseline patient characteristics, procedural factors, medications, and center. The unadjusted and adjusted risks for clinical events are expressed as hazard ratios with their 95% confidence intervals. The detailed methods of the multivariate analyses are described in Supplementary Methods .
The landmark analysis based on DAPT use at 4 months after the index PCI was conducted as described previously. Eligible patients for the landmark analysis were those who were alive and free from stroke, MI, ST, and major bleeding at the 4-month landmark point. Taking a 1-month window period, the 4-month landmark point was selected because DAPT for at least 3 months had been recommended after implantation of sirolimus-eluting stent, which was the most commonly used DES in this study population.
All analyses were conducted by 2 physicians (KG and KN) and a statistician (TMo) with the use of SAS 9.2 and JMP 7.0 (SAS Institute Inc., Cary, North Carolina). All the statistical analyses were 2-tailed, and probability values <0.05 were considered statistically significant.
Results
Among the entire 12,716 study patients, 1,057 patients (8.3%) had AF. Baseline characteristics of the entire study population comparing patients with AF and no-AF patients are listed in Supplementary Table 1 . During the median follow-up of 5.1 years (Q1 to Q3; 4.3 to 5.9), a total of 2,065 patients died and 800 patients had strokes. Cumulative 5-year incidence of stroke was significantly higher in patients with AF than in no-AF patients (12.8% and 5.8%, p <0.0001; Figure 2 ). After adjusting for confounders, the excess risk of stroke for patients with AF relative to no-AF patients remained significant ( Table 1 ).
| Clinical Outcome | No. of Events (5-Year Cumulative Incidence) | p Value | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| (A) AF versus No-AF | AF (n = 1057) | No-AF (n = 11659) | ||||
| Stroke ∗ | 134 (12.8%) | 666 (5.8%) | <0.0001 | 2.47 (2.05–2.97) | 2.00 (1.65–2.43) | <0.0001 |
| Ischemic or unspecified † | 113 (10.9%) | 515 (4.4%) | <0.0001 | 2.68 (2.19–3.29) | 2.16 (1.74–2.67) | <0.0001 |
| Hemorrhagic | 23 (2.4%) | 164 (1.5%) | 0.02 | 1.69 (1.09–2.61) | 1.40 (0.89–2.21) | 0.15 |
| All-caused death | 312 (27.6%) | 1753 (13.9%) | <0.0001 | 2.18 (1.93–2.46) | 1.43 (1.26–1.62) | <0.0001 |
| Major bleeding | 166 (16.7%) | 1200 (10.2%) | <0.0001 | 1.66 (1.41–1.95) | 1.22 (1.03–1.44) | 0.02 |
| Myocardial infarction | 61 (6.5%) | 559 (4.7%) | 0.0497 | 1.30 (1.00–1.70) | 1.22 (0.93–1.61) | 0.15 |
| Stent thrombosis ‡ | 18 (1.7%) | 185 (1.6%) | 0.62 | 1.13 (0.70–1.83) | 1.17 (0.71–1.93) | 0.53 |
| (B) OAC versus No-OAC | OAC (n = 506) | No-OAC (n = 551) | ||||
| Stroke ∗ | 68 (13.8%) | 66 (11.8%) | 0.49 | 1.13 (0.80–1.58) | 1.20 (0.83–1.73) | 0.34 |
| Ischemic | 57 (11.5%) | 56 (10.3%) | 0.59 | 1.11 (0.77–1.60) | 1.22 (0.82–1.83) | 0.33 |
| Hemorrhagic | 13 (3.2%) | 10 (1.6%) | 0.42 | 1.41 (0.62–3.21) | 2.68 (0.78–9.24) | 0.12 |
| All–caused death | 142 (25.5%) | 170 (29.4%) | 0.35 | 0.90 (0.72–1.12) | 0.93 (0.72–1.19) | 0.56 |
| Major bleeding | 74 (16.2%) | 92 (17.1%) | 0.29 | 0.85 (0.63–1.15) | 0.81 (0.58–1.13) | 0.21 |
| Myocardial infarction | 17 (4.5%) | 44 (8.5%) | 0.001 | 0.40 (0.23–0.71) | 0.39 (0.21–0.74) | 0.004 |
| Stent thrombosis ‡ | 4 (1.0%) | 14 (2.5%) | 0.03 | 0.31 (0.10–0.93) | 0.14 (0.03–0.82) | 0.03 |
| (C) TTR ≥65% versus <65% | TTR ≥65% (n = 154) | TTR <65% (n = 255) | ||||
| Stroke ∗ | 11 (6.9%) | 36 (15.1%) | 0.01 | 0.44 (0.22–0.87) | 0.37 (0.16–0.86) | 0.02 |
| Ischemic | 8 (4.9%) | 30 (12.6%) | 0.01 | 0.38 (0.18–0.84) | 0.30 (0.11–0.81) | 0.02 |
| Hemorrhagic | 4 (3.1%) | 7 (3.4%) | 0.85 | 0.89 (0.26–3.02) | — § | — |
| All-caused death | 31 (17.8%) | 74 (25.9%) | 0.02 | 0.62 (0.41–0.94) | 0.86 (0.51–1.43) | 0.56 |
| Major bleeding | 17 (10.4%) | 44 (19.6%) | 0.06 | 0.59 (0.34–1.04) | 0.50 (0.25–1.01) | 0.053 |
| Myocardial infarction | 4 (3.1%) | 10 (5.0%) | 0.41 | 0.62 (0.19–1.97) | — § | — |
| Stent thrombosis ‡ | 1 (0.9%) | 3 (1.3%) | 0.57 | 0.53 (0.06–5.09) | — § | — |
∗ The sum of the numbers of ischemic (or unspecified) and hemorrhagic stroke events is not necessarily equal to the number of overall stroke events because of patients with multiple events.
† Only 8 out of 800 strokes (1.0%) were unspecified because of lack of imaging information, all of which were in no-AF patients.
‡ Academic Research Consortium definite.
Among 1,057 patients with AF, although a large number of patients had a CHADS 2 score of ≥2, only 506 patients (47.9%) received OAC with warfarin ( Table 2 ).
| Characteristic | AF Patients (n = 1057) | OAC Group (n = 506) | No-OAC Group (n = 551) | p Value |
|---|---|---|---|---|
| Age (years) | 72.5 ± 9.3 | 72.0 ± 8.8 | 73.0 ± 9.7 | 0.04 |
| Age ≥75 years | 477 (45.1%) | 212 (41.9%) | 265 (48.1%) | 0.04 |
| Male | 752 (71.1%) | 383 (75.7%) | 369 (67.0%) | 0.002 |
| AF type | ||||
| Paroxysmal | 652 (61.7%) | 247 (48.8%) | 405 (73.5%) | <0.0001 |
| Persistent/permanent | 302 (28.6%) | 207 (40.9%) | 95 (17.2%) | |
| Unknown | 103 (9.7%) | 52 (10.3%) | 51 (9.3%) | |
| Body mass index <25.0 kg/m 2 | 766 (72.5%) | 356 (70.4%) | 410 (74.4%) | 0.14 |
| Acute myocardial infarction | 392 (37.1%) | 168 (33.2%) | 224 (40.7%) | 0.01 |
| Hypertension | 902 (85.3%) | 435 (86.0%) | 467 (84.8%) | 0.58 |
| Diabetes mellitus | 362 (34.2%) | 177 (35.0%) | 185 (33.6%) | 0.63 |
| On insulin therapy | 60 (5.7%) | 25 (4.9%) | 35 (6.4%) | 0.32 |
| Current smoker | 237 (22.4%) | 118 (23.3%) | 119 (21.6%) | 0.50 |
| Heart failure | 417 (39.5%) | 201 (39.7%) | 216 (39.2%) | 0.86 |
| Shock at presentation | 98 (9.3%) | 39 (7.7%) | 59 (10.7%) | 0.09 |
| Multivessel coronary artery disease | 529 (50.0%) | 238 (47.0%) | 291 (52.8%) | 0.06 |
| Ejection fraction | 55.4 ± 14.1 | 54.4 ± 14.4 | 56.4 ± 13.8 | 0.04 |
| Mitral regurgitation grade 3/4 | 109 (10.3%) | 53 (13.8%) | 56 (15.2%) | 0.59 |
| Prior myocardial infarction | 128 (12.1%) | 62 (12.3%) | 66 (12.0%) | 0.89 |
| Prior stroke | 196 (18.5%) | 96 (19.0%) | 100 (18.2%) | 0.73 |
| Prior intracranial bleeding | 27 (2.6%) | 7 (1.4%) | 20 (3.6%) | 0.02 |
| Peripheral vascular disease | 87 (8.2%) | 53 (10.5%) | 34 (6.2%) | 0.01 |
| eGFR <30, not on dialysis | 59 (5.6%) | 28 (5.5%) | 31 (5.6%) | 0.95 |
| Dialysis | 48 (4.5%) | 19 (3.8%) | 29 (5.3%) | 0.24 |
| Anemia (Hb <11.0 g/dl) | 153 (14.5%) | 56 (11.1%) | 97 (17.6%) | 0.002 |
| Platelet <100 × 10 9 /L 3 | 30 (2.8%) | 13 (2.6%) | 17 (3.1%) | 0.61 |
| Chronic obstructive pulmonary disease | 43 (4.1%) | 20 (4.0%) | 23 (4.2%) | 0.86 |
| Liver cirrhosis | 30 (2.8%) | 17 (3.4%) | 13 (2.4%) | 0.33 |
| Malignancy | 108 (10.2%) | 47 (9.3%) | 61 (11.1%) | 0.34 |
| CHADS 2 score | 2.4 ± 1.3 | 2.4 ± 1.2 | 2.4 ± 1.3 | 0.85 |
| CHADS 2 score ≥2 | 795 (75.2%) | 389 (76.9%) | 406 (73.7%) | 0.23 |
| CHA 2 DS 2 -VASc score | 4.5 ± 1.5 | 4.5 ± 1.5 | 4.6 ± 1.6 | 0.29 |
| Stent use | 959 (90.7%) | 447 (88.3%) | 512 (92.9%) | 0.01 |
| DES use | 506 (47.9%) | 264 (52.2%) | 242 (43.9%) | 0.007 |
| Aspirin | 1037 (98.1%) | 495 (97.8%) | 542 (98.4%) | 0.52 |
| Thienopyridine | 1005 (95.1%) | 473 (93.5%) | 532 (96.6%) | 0.02 |
| DAPT | 989 (93.6%) | 465 (91.9%) | 524 (95.1%) | 0.03 |
| Warfarin | 506 (47.9%) | 506 (100%) | 0 (0%) | — |
| Statins | 430 (40.7%) | 210 (41.5%) | 220 (39.9%) | 0.60 |
| Beta-blockers | 403 (38.1%) | 221 (43.7%) | 182 (33.0%) | 0.0004 |
| ACE-I/ARB | 646 (61.1%) | 328 (64.8%) | 318 (57.7%) | 0.02 |
| Proton pump inhibitors | 310 (29.3%) | 132 (26.1%) | 178 (32.3%) | 0.03 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


