Shunts at ventricular or great vessel level
Although most patients with one of these malformations are asymptomatic, poor growth and symptoms of congestive cardiac failure occur in the 5% of patients with greatly increased blood flow. A tendency for frequent respiratory infections and episodes of pneumonia is common in those with a large shunt.
In this chapter, the factors governing flow in a ventricular septal defect and in a patent ductus arteriosus will be discussed in greater detail. This information should be carefully studied and mastered, as it can be applied for understanding more complex anomalies that also have a communication between the two sides of the circulation.
Ventricular septal defect
Ventricular septal defect (Figure 4.1), the most frequently occurring congenital cardiac anomaly, is present in at least one-fourth of all patients. Overall, a ventricular septal defect is a component in half of all patients with a cardiac malformation. An example is the VSD in tetralogy of Fallot.
Isolated ventricular septal defects causing clinical concern are most frequently located in the perimembranous portion of the ventricular septum. Less frequently they are found either above the crista supraventricularis or in the muscular portion of the septum.
Small defects in the muscular ventricular septum create characteristic murmurs in neonates and young infants as pulmonary resistance falls. It is the most common cardiac “defect” (reported in as many as 5% of neonates, as detected by echocardiography). Most small muscular defects close spontaneously within the first few months of life. Through the defect, blood shunts from the left to the right ventricle. When the size of the defect approaches the size of the aortic annulus, flow is governed by the relative pulmonary and systemic vascular resistances. When the defect is smaller, blood flows from the left to the right ventricle because of the higher left ventricular systolic pressure.
Because two physiologic mechanisms influence the shunt, the clinical findings, natural history, and operative considerations for the two different sizes (large and small) of ventricular septal defects will be considered separately.
Large ventricular septal defect
In patients whose ventricular septal defect approaches the diameter of the aortic annulus, the resistance to outflow from the heart is determined primarily by the caliber of the arterioles of the systemic and pulmonary vascular beds.
Since the systemic arterioles have a thick muscular coat and narrow lumen and the pulmonary arterioles have a thin coat and wide lumen, the systemic resistance is greater than the pulmonary resistance.
Since the flow through a large defect is governed by resistances, any condition that increases resistance to left ventricular outflow, such as coarctation of the aorta or aortic stenosis, increases the magnitude of the left-to-right shunt, whereas any abnormality that obstructs right ventricular outflow, such as coexistent pulmonary stenosis, as in tetralogy of Fallot, or pulmonary arteriolar disease, decreases the magnitude of the left-to-right shunt. If the resistance to right ventricular outflow exceeds the resistance to left ventricular outflow, the shunt is in a right-to-left direction.
Although this sequence occurs in every individual, this decrease in pulmonary vascular resistance has profound effects on patients with a ventricular septal defect. In those with a large ventricular septal defect, the medial layer does not undergo regression either as quickly or to the extent of an normal individual. Therefore, at any age the pulmonary vascular resistance is higher than normal yet lower than the systemic resistance.
In patients with a large isolated ventricular septal defect, the systolic pressures in both ventricles and both great vessels are the same, with the right-sided systolic pressures elevated to the same levels as those normally present on the left side of the heart. Because the aortic systolic pressure is regulated at a constant level by baroreceptors, the pulmonary artery pressure (P) is also relatively fixed. According to P = RP × QP, as the pulmonary vascular resistance (RP) falls, the volume of pulmonary blood flow (QP) increases. This occurrence contrasts with the events that occur in an infant without a shunt, who has constant pulmonary blood flow (QP); therefore, according to P = RP × QP, as pulmonary resistance (RP) falls following birth, so does the pulmonary arterial pressure (P).
Among patients with a large ventricular septal defect, as the pulmonary resistance falls as a consequence of the maturation of the pulmonary vessels, the volume of pulmonary blood flow increases no matter what the level of the pulmonary arterial pressure is. At birth, flow through the defect is limited, but as the neonate and then young infant grows, the pulmonary blood flow progressively increases.
Large ventricular septal defects place two major hemodynamic loads upon the ventricles: increased pressure load on the right ventricle and increased volume load on the left ventricle.
In a large defect, the right ventricle develops a level of systolic pressure equal to that of the left ventricle. The right ventricular workload is proportional to the level of pulmonary arterial pressure (P = R × Q); pulmonary arterial hypertension results from either increased pulmonary arterial resistance or increased pulmonary blood flow. Regardless of the origin of pulmonary hypertension, the right ventricle is thick-walled; but its state does not really change from fetal life when it also developed high levels of pressure. Since the pressure remains elevated postnatally, the normal evolution of the right ventricle to a thin-walled, crescent-shaped chamber does not occur. The right ventricle is able to tolerate and to maintain these levels of pressure without the development of cardiac failure.
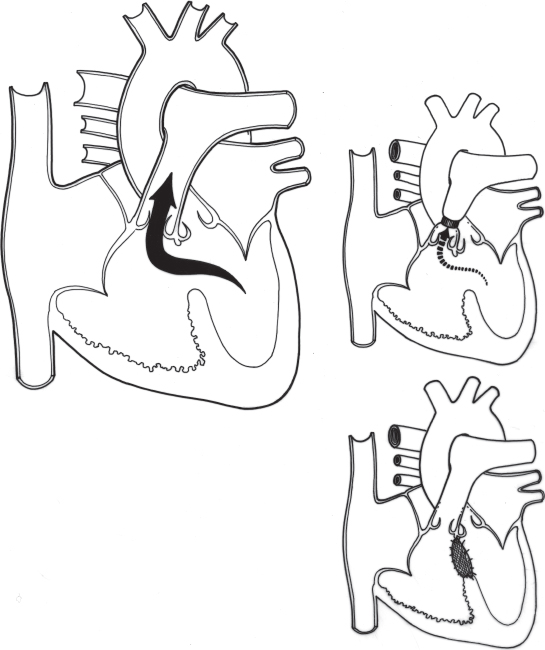
In a large ventricular septal defect and left-to-right shunt, volume overload of the left ventricle exists because this chamber not only maintains the systemic blood flow but also ejects blood through the ventricular septal defect into the pulmonary vascular bed. When the ventricles contract, the flow from the left ventricle through the ventricular septal defect is directed almost entirely into the pulmonary artery, and the right ventricle has little additional volume load. The augmented pulmonary blood flow returns through the left atrium to the left ventricle.
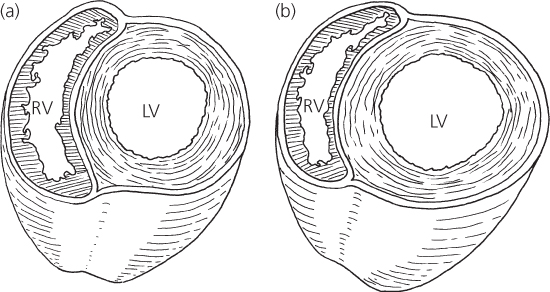
To accommodate the increased pulmonary venous return, the left ventricle dilates (Figure 4.3). As dilation occurs, the radius and circumference of the left ventricle increase and the myocardial fibers lengthen. Both the Laplace and Starling laws describe this relationship.
Figure 4.3 Cross-section through ventricles. (a) Normal contour and (b) dilated left ventricle in ventricular septal defect. LV, left ventricle; RV, right ventricle.
The signs and symptoms of a large ventricular septal defect vary with the relative vascular resistances and the volume of pulmonary blood flow. In evaluating a patient with a large ventricular septal defect, the physician should seek diagnostic information that permits definition of pulmonary blood flow (QP) and pulmonary artery pressure (P) so that pulmonary vascular resistance (RP) can be estimated.
History
In many patients with a large ventricular septal defect, the murmur may not be heard until the first postnatal visit. By that age, the pulmonary vascular resistance has fallen sufficiently that enough blood flows through the defect to generate the murmur.
Patients with a large defect develop congestive cardiac failure by 2–3 months of age. By this time, the pulmonary arterioles have matured sufficiently to permit a large volume of pulmonary blood flow. As a consequence, left ventricular dilation develops and results in cardiac failure and its symptoms of tachypnea, slow weight gain, and poor feeding.
Physical examination
Pansystolic murmur
The classic auscultatory finding is a loud pansystolic murmur heard best in the third and fourth left intercostal spaces. Usually associated with a thrill, the murmur is widely transmitted. The murmur begins with the first heart sound and includes the isovolumetric contraction period of the cardiac cycle. Since the ventricles are in communication, blood shunts from the left to the right ventricle from the onset of systole. The murmur usually lasts until the second heart sound. The loudness of the murmur does not directly relate to the size of the defect; loudness depends on other factors, such as volume of blood flow through the defect. However, large defects tend not necessarily to produce loud pansystolic murmurs.
Mid-diastolic murmur
In patients with a large ventricular septal defect and a large volume of pulmonary blood flow, the volume of pulmonary venous blood crossing the mitral valve from the left atrium into the left ventricle during diastole is greatly increased. When the volume of blood flow across the mitral valve exceeds twice normal, a mid-diastolic inflow murmur may be heard, often following the third heart sound. Low pitched, it is best heard at the cardiac apex. The loudness roughly parallels the volume of pulmonary blood flow.
Loud P2
Patients with a large ventricular septal defect have pulmonary hypertension related to various combinations of pulmonary blood flow and increased pulmonary vascular resistance. Regardless of etiology, pulmonary hypertension is indicated by an increased loudness of the pulmonary component of the second heart sound. The louder the pulmonary component, the higher is the pulmonary arterial pressure.
In the presence of an apical diastolic murmur, the loud pulmonic valve closure primarily relates to increased pulmonary flow. The absence of a mitral diastolic murmur indicates that the pulmonary hypertension is secondary to increased pulmonary vascular resistance.
Clinical evidence of cardiomegaly
Cardiomegaly is found in patients with increased pulmonary blood flow; it is indicated by a laterally and inferiorly displaced cardiac apex and/or a precordial bulge.
Congestive cardiac failure
Tachypnea, tachycardia, and dyspnea (especially with poor feeding and diaphoresis increasing during feeding in infants) suggest congestive cardiac failure. Cardiomegaly and hepatomegaly support the diagnosis. Peripheral edema and abnormal lung sounds are not typical signs of congestive heart failure in infants.
Electrocardiogram
The electrocardiogram reflects the types of hemodynamic load placed upon the ventricles: left ventricular volume overload related to increased pulmonary blood flow and right ventricular pressure overload related to pulmonary hypertension.
Chest X−ray
The chest X-ray (Figure 4.6) shows normal-appearing pulmonary vasculature at birth, but soon thereafter the vascularity increases. The radiographic appearance of the heart varies according to the magnitude of the shunt and the level of pulmonary arterial pressure. Ranging from normal to markedly enlarged, the size varies directly with the magnitude of the shunt.
Figure 4.6 Chest X-ray in ventricular septal defect. Cardiomegaly and increased pulmonary vascular markings. The lateral view shows left atrial enlargement, outlined by barium within the esophagus.
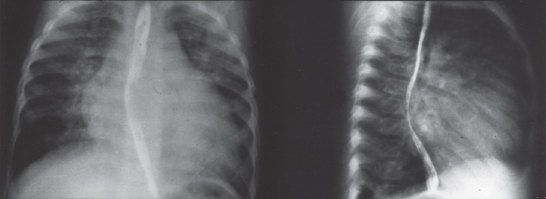
The cardiac enlargement results from enlargement of both the left atrium and the left ventricle from the increased flow. The left atrium is a particularly valuable indicator of pulmonary blood flow because this chamber is easily assessed on a lateral projection. By itself the right ventricular hypertrophy does not contribute to cardiac enlargement. The pulmonary artery can be enlarged from either the volume of pulmonary blood flow or pulmonary hypertension. There is no characteristic contour of the heart in ventricular septal defect.
Natural history
An uncorrected large ventricular septal defect may follow one of three clinical courses.
Pulmonary vascular disease
Pulmonary vascular disease may develop. The initiating factors for the development of medial hypertrophy and later intimal proliferation are unknown, but they are probably related to the arterioles being subjected to high levels of pressure and, to a lesser extent, to elevated blood flow. The pulmonary arteriolar changes can develop in pulmonary arterioles of children as young as 1 year of age. The early changes of medial hypertrophy are generally reversible if the ventricular septal defect is closed, but the intimal changes are permanent. The pathologic changes of the pulmonary arterioles usually progress unless the course is interrupted by operation. Children with Down syndrome appear to develop irreversible (or, if reversible, a more reactive and problematic) elevation of pulmonary vascular resistance within the first 6 months of life.
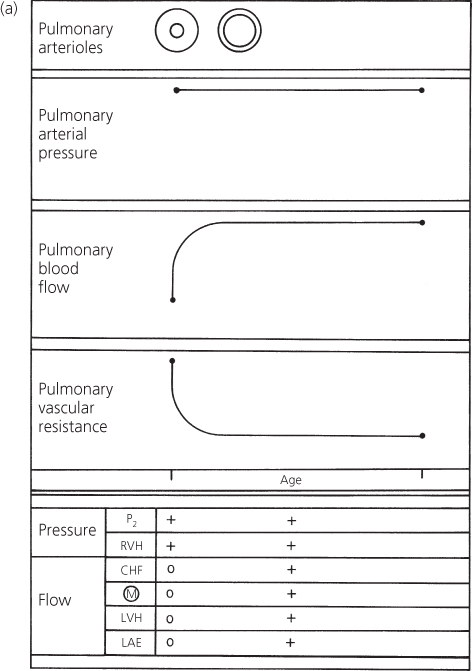
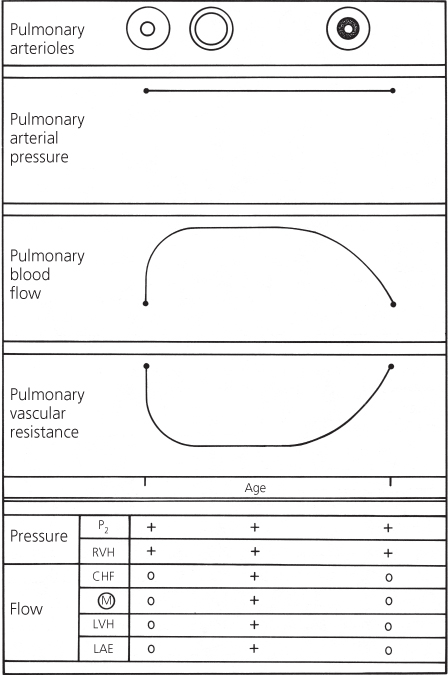
The result of these pulmonary arteriolar changes is progressive elevation of pulmonary vascular resistance (Figure 4.7). The pulmonary arterial pressure does not increase, but instead remains constant because the ventricles are in free communication. Therefore, the volume of pulmonary blood flow decreases.
Figure 4.7 Changes in pulmonary arterial pressure, pulmonary blood flow, and pulmonary vascular resistance in a patient with a large ventricular septal defect who develops pulmonary vascular disease. Correlation with major clinical findings reflecting pulmonary arterial pressure and pulmonary blood flow. CHF, congestive heart failure; LAE, left atrial enlargement; LVH, left ventricular hypertrophy; M, murmur; P2, pulmonary component of second heart sound; RVH, right ventricular hypertrophy.
Eventually, the pulmonary vascular resistance may exceed systemic vascular resistance, at which time the shunt becomes right-to-left through the defect and cyanosis develops (Eisenmenger syndrome).
The progressive rise in pulmonary vascular resistance can be followed clinically by observing the changes in the secondary features of ventricular septal defect. Those features reflecting elevated pulmonary arterial pressure, right ventricular hypertrophy, and loudness of the pulmonary component remain constant, whereas those reflecting pulmonary blood flow change (Figure 4.7).
The clinical findings reflecting the excessive flow through the left side of the heart gradually disappear. Congestive cardiac failure lessens, the diastolic murmur fades, the electrocardiogram no longer shows the left ventricular hypertrophy, and the cardiac size becomes smaller on a chest X-ray. The heart size eventually becomes normal when the total volume of blood flow is normal. The right ventricle is hypertrophied, but this does not cause enlargement. For many patients with cardiac disease, the disappearance of congestive cardiac failure and the presence of a normal heart size are favorable; but in a large ventricular septal defect the changes are ominous.
Infundibular pulmonary stenosis
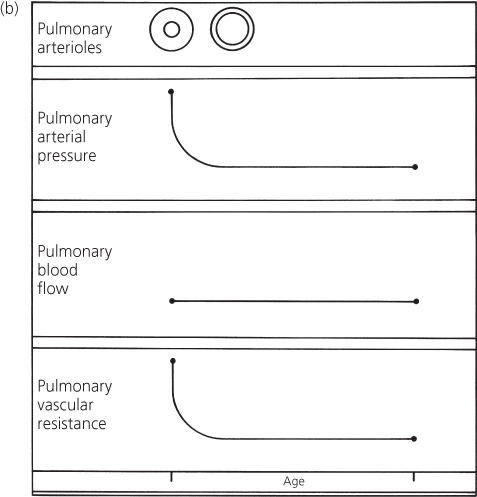
Infundibular pulmonary stenosis may develop. In certain patients with a large ventricular septal defect, infundibular stenosis develops and progressively narrows the right ventricular outflow tract. The stenotic area presents a major resistance to outflow to the lungs; the pulmonary vascular resistance is often normal (Figure 4.8). The shunt in these patients is influenced by the relationship between the systemic vascular resistance and the resistance that is imposed by the infundibular stenosis. Eventually, the latter may exceed the former so that the shunt becomes right-to-left and cyanosis develops. The clinical picture of these patients then resembles tetralogy of Fallot.
Figure 4.8 Changes in pulmonary arterial pressure, pulmonary blood flow, and pulmonary vascular resistance in a patient with a large ventricular septal defect who develops infundibular pulmonary stenosis. Correlation with major clinical findings reflecting pulmonary arterial pressure and pulmonary blood flow. Dashed line indicates resistance imposed by infundibular stenosis. CHF, congestive heart failure; LAE, left atrial enlargement; LVH, left ventricular hypertrophy; M, murmur; P2, pulmonary component of second heart sound; RVH, right ventricular hypertrophy.
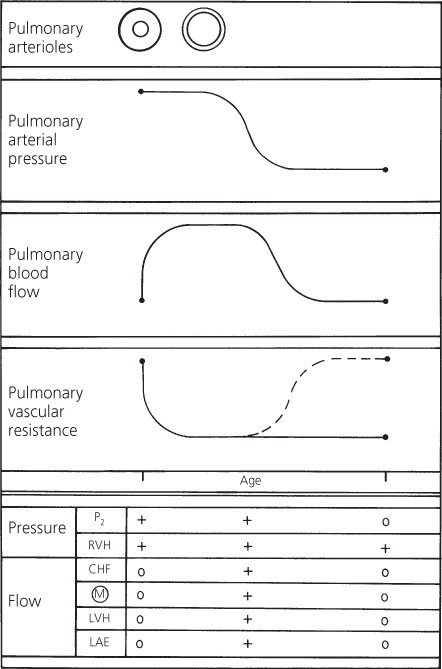
In these patients, the loudness of the pulmonary component becomes normal or is reduced and delayed, but right ventricular hypertrophy persists because the right ventricle is still developing a systemic level of pressure. The features related to pulmonary blood flow – congestive cardiac failure, apical diastolic murmur, left ventricular hypertrophy on the electrocardiogram, cardiomegaly, and left atrial enlargement on a chest X-ray – disappear as the pulmonary blood flow is reduced.
Regardless of whether the resistance to pulmonary blood flow resides in the infundibulum or the pulmonary arterioles, the hemodynamic effects are similar; but the prognosis is different.
Spontaneous closure
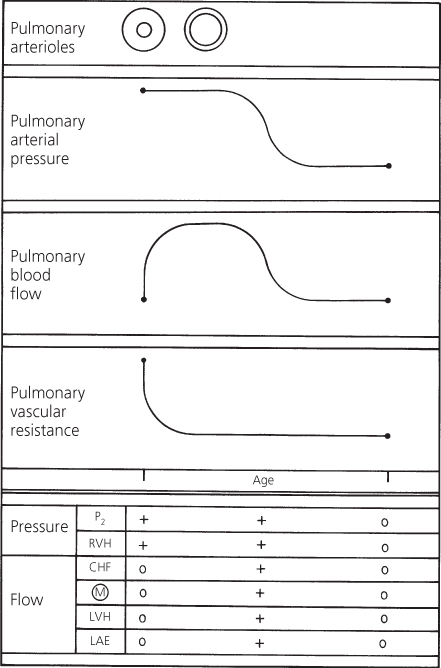
Spontaneous closure of the ventricular septal defect may occur. The exact incidence of spontaneous closure is unknown, but up to 5% of large ventricular septal defects and at least 75% of small defects undergo spontaneous closure; others become smaller. The spontaneous closure occurs by two basic mechanisms: either by adherence of the septal leaflet of the tricuspid valve to the ventricular septum which occludes the defect or by closure of a muscular defect by ingrowth of myocardium and then fibrous proliferation. The perimembranous defect may become smaller by the septal tricuspid valve leaflet creating a mobile and partially restrictive so-called aneurysm of the membranous septum.
Most instances of spontaneous closure occur by 3 years of age, but may close in adolescents or even adulthood when the pulmonary vascular resistance is still near normal levels.
As the closure of the ventricular septal defect occurs, the systolic murmur softens, and of the secondary features that reflect pulmonary arterial pressure (Figure 4.9), the pulmonary component becomes normal and the right ventricular hypertrophy disappears. Those features that reflect increased pulmonary blood flow also gradually disappear. Thus, eventually, the systolic murmur disappears and no residual cardiac abnormalities exist, although the heart may remain large for some months. Some liken the gradual resolution of cardiomegaly to the process of a patient “growing into” their own heart size, rather than calling it an active reduction in heart size.
Figure 4.9 Changes in pulmonary arterial pressure, pulmonary blood flow, and pulmonary vascular resistance in a patient with a large ventricular septal defect that undergoes spontaneous closure. Correlation with major clinical findings reflecting pulmonary arterial pressure and pulmonary blood flow. CHF, congestive heart failure; LAE, left atrial enlargement; LVH, left ventricular hypertrophy; M, murmur; P2, pulmonary component of second heart sound; RVH, right ventricular hypertrophy.
Echocardiogram
A large ventricular septal defect appears as an area of “dropout” within the septum by cross-sectional two-dimensional (2D) echocardiography.
Perimembranous infracristal defects appear near the tricuspid valve septal leaflet and the right aortic valve cusp.
Small defects, especially those within the trabecular (muscular) septum, may not be apparent by 2D, but color Doppler demonstrates a multicolored jet traversing the septum, representing the turbulent shunt from left to right ventricle.
Inlet ventricular septal defect, located near the AV valves, is seen in atrioventricular septal defect.
The maximum velocity of the blood traversing the defect, determined by spectral Doppler, is used to estimate the interventricular pressure difference. Large defects that lead to high right ventricular systolic pressure are reflected as low-velocity flow across the defect. In a small defect with normal right ventricular systolic pressure, the shunt is of high velocity, reflecting the large interventricular pressure difference. Small ventricular septal defects in neonates may have low-velocity flow, indicating that pulmonary resistance and right ventricular pressure have not yet fallen. Low-velocity shunt, or right-to-left shunt, is seen in older patients with pulmonary vascular obstructive disease or right ventricular outflow obstruction.
In patients with a large ventricular septal defect, 2D echocardiography reveals left atrial and left ventricular enlargement. Left ventricular systolic function may appear hyperdynamic because of the increased stroke volume associated with a large ventricular septal defect. The pulmonary systolic pressure can be determined by analysis of the Doppler signal that regurgitates through the tricuspid valve.
Cardiac catheterization
Cardiac catheterization may be indicated in patients with multiple ventricular septal defects and congestive cardiac failure, elevated pulmonary vascular resistance, or associated cardiovascular anomalies. The purposes of the procedure are to define the hemodynamics, to identify coexistent cardiac anomalies, and to localize the site(s) of the ventricular septal defect(s).
A large increase in oxygen saturation is found at the right ventricular level. The pulmonary arterial and right ventricular systolic pressures are identical with those in the aorta and the left ventricle. If the pulmonary vascular resistance is increased, the increase in oxygen saturation at the right ventricular level is not as large as when it is lower. Pulmonary arterial pressure remains at the same level. The left-to-right shunt is not as large.
Left ventriculography is indicated to locate the position of the ventricular septal defect(s) because location influences operative repair. Aortography may also be performed to exclude a coexistent patent ductus arteriosus, which can be a silent partner to the ventricular septal defect.
Operative considerations
Patients with a large ventricular septal defect and congestive cardiac failure should be treated with diuretics, inotropes, and/or afterload reduction and with aggressive nutritional support (discussed in Chapter 11). Fluid restriction (which also means caloric restriction) is usually counterproductive. Although these measures improve the clinical status, many patients frequently show persistent findings of cardiac failure, indicating a need for operative treatment. Two operative procedures are available.
Corrective operation
Corrective operation for closure of the ventricular septal defect is indicated in infancy for patients with persistent cardiac failure and pulmonary hypertension. Cardiopulmonary bypass is instituted, the right atrium is opened, and, by working through the tricuspid valve, the ventricular septal defect is closed using a patch of Dacron or pericardium. This technique avoids a transmural scar in the ventricular myocardium. The operative mortality risk in infants is less than 0.25%. The long-term results of the procedure are excellent; virtually no patients who had normal or reactive pulmonary vascular resistance preoperatively develop late pulmonary vascular obstructive disease. Almost no patients develop endocarditis or arrhythmia late postoperatively.
Banding of the pulmonary artery
Banding of the pulmonary artery is a palliative procedure that causes an increase in the resistance to blood flow into the lungs. Therefore, the pulmonary artery pressure and volume of blood flow returning to the left side of the heart are reduced, improving congestive cardiac failure. Pulmonary artery deformity and stenosis may persist after removal of the band.
Because the risk for operative ventricular septal defect closure is low (usually less than that for banding and subsequent reoperation for debanding with defect closure), corrective surgery is preferable. For some cardiac malformations with a large ventricular communication (e.g. single ventricle), pulmonary artery banding is indicated as temporary or permanent palliation.
Small or medium ventricular septal defects
The size of ventricular septal defects varies considerably. The previous section discussed those defects whose diameter approached the size of the aortic annulus. This section discusses smaller ventricular septal defects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree