Anomalies of the Pulmonary Veins
David W. Brown
Tal Geva
Anomalies of the pulmonary veins vary widely in their anatomic spectrum and clinical presentation, course, and outcome. As with other types of congenital heart disease, advents in diagnosis, cardiovascular surgery in newborns and infants, and transcatheter therapy have heightened the importance of early recognition, accurate diagnosis, and better understanding of abnormalities of the pulmonary veins.
Anatomy
Pulmonary venous anomalies may be categorized as anomalous connections, anomalous drainage with normal connections, stenotic connections, and abnormal numbers of pulmonary veins.
Anomalous Connections and Drainage
One or more of the pulmonary veins may connect anomalously to one or more of the systemic veins. The condition is termed totally anomalous pulmonary venous connection (TAPVC) if all the veins connect anomalously, and partially anomalous pulmonary venous connection (PAPVC) if one or more, but not all, of the veins connect anomalously.
The physiologic consequence of anomalous pulmonary venous connection is usually anomalous pulmonary venous drainage. It is also possible, however, to have normal pulmonary venous connections with abnormal drainage. Examples of this condition include a common atrium and malposition of the septum primum. Although the pulmonary veins in these anomalies connect normally (e.g., into the back wall of the atria between the right and left horns of the sinus venosus), either complete absence of the interatrial septum or malattachment of the septum primum can lead to anomalous drainage into the morphologic right atrium (RA). When some or all of the pulmonary veins drain anomalously into the RA or its tributaries without being abnormally connected, the terms partially anomalous pulmonary venous drainage (PAPVD) or totally anomalous pulmonary venous drainage (TAPVD) with normal pulmonary venous connections are used. Therefore, the terms pulmonary venous connections and pulmonary venous drainage should not be used synonymously.
Stenotic Connections
Stenosis in one or more of the pulmonary veins or in the common pulmonary vein may occur. Veins with normal connections may exhibit stenoses, varying from stenosis of one or more of the individual pulmonary veins to cor triatriatum. These produce obstruction to pulmonary venous return to the left atrium (LA). Anomalously connected veins may be stenosed, resulting in obstruction to pulmonary venous return to the RA. The physiologic feature common to these stenotic connections is obstruction of egress of pulmonary venous blood.
Abnormal Numbers of Pulmonary Veins
Normally, there are two right and two left pulmonary veins. The most common variation is the presence of a single pulmonary vein on either the right or left side, with a prevalence of about 24% in anatomic studies (1). Rarely, all pulmonary veins enter a common pulmonary vein that drains into the LA. A single left pulmonary vein was found more frequently than a single right pulmonary vein (1). A single common pulmonary vein, usually without stenosis, occurs almost exclusively in cases of visceral heterotaxy with asplenia. It also is possible to have an increased number of normally connecting pulmonary veins. The prevalence of a third pulmonary vein on either the right or left side is 1.6% to 2% (1). A fourth or even a fifth vein infrequently is present. An abnormal number of pulmonary veins impose no physiologic handicap.
Embryology
A classification of pulmonary venous anomalies based on embryologic principles introduces a unifying concept to the consideration of these anatomically and physiologically diverse conditions. Review of the embryologic development of the pulmonary venous system is a prerequisite.
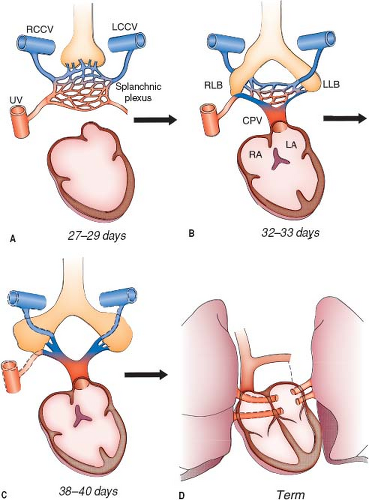
In the human embryo, the primordia of the lungs, larynx, and tracheobronchial tree are derived by a division of the foregut. In their early stages of development, the lungs are enmeshed by the vascular plexus of the foregut, the splanchnic plexus. As pulmonary differentiation progresses, part of the splanchnic plexus forms the pulmonary vascular bed. At this stage, there is no direct connection to the heart. Instead, the pulmonary vascular bed shares the routes of drainage of the splanchnic plexus (i.e., umbilicovitelline and cardinal systems of veins) (Fig. 35.1A). Subsequently, the intraparenchymal pulmonary veins connect with the LA by establishing a connection with the common pulmonary vein, which evaginates from the LA (2).
No unanimous opinion about the site of development of the common pulmonary vein has been attained. Some investigators believe the common pulmonary vein originates from an evagination in the sinoatrial region of the heart (3). Others believe that the common pulmonary vein starts from a confluence of vessels
from the pulmonary plexus (1). According to a third opinion, the beginning of the common pulmonary vein occurs by the confluence of capillaries that grow into the mesocardium, located between the lung buds and the heart. Nevertheless, it is generally accepted that, by the end of the first month of gestation, the common pulmonary vein can be identified as a vessel draining the pulmonary plexus and entering the sinoatrial portion of the heart. The site of entry is cephalad to the junction of the left and right horns of the sinus venosus and to the left of the developing septum primum (Fig. 35.1B) (3,4).
from the pulmonary plexus (1). According to a third opinion, the beginning of the common pulmonary vein occurs by the confluence of capillaries that grow into the mesocardium, located between the lung buds and the heart. Nevertheless, it is generally accepted that, by the end of the first month of gestation, the common pulmonary vein can be identified as a vessel draining the pulmonary plexus and entering the sinoatrial portion of the heart. The site of entry is cephalad to the junction of the left and right horns of the sinus venosus and to the left of the developing septum primum (Fig. 35.1B) (3,4).
When the direct connection to the heart is established, the initial communications between the pulmonary portion of the
splanchnic plexus and the cardinal and umbilicovitelline systems are, for the most part, obliterated. The pulmonary vascular bed then drains via four individual major pulmonary veins into the common pulmonary vein, which in turn empties into the LA (Fig. 35.1C). The common pulmonary vein is a transient anatomic structure. By a process of differential growth, it becomes incorporated into the LA, resulting in the ultimate anatomic arrangement wherein the four individual pulmonary veins connect separately and directly to the LA (Fig. 35.1D).
splanchnic plexus and the cardinal and umbilicovitelline systems are, for the most part, obliterated. The pulmonary vascular bed then drains via four individual major pulmonary veins into the common pulmonary vein, which in turn empties into the LA (Fig. 35.1C). The common pulmonary vein is a transient anatomic structure. By a process of differential growth, it becomes incorporated into the LA, resulting in the ultimate anatomic arrangement wherein the four individual pulmonary veins connect separately and directly to the LA (Fig. 35.1D).
Imperfect development of the common pulmonary vein provides embryologic basis for most anomalies of the pulmonary veins. The following aberrations of development of the common pulmonary vein explain these anomalies and are used as a means of classifying them (Table 35.1).
Early Atresia of the Common Pulmonary Vein while Pulmonary–Systemic Venous Connections are Still Present
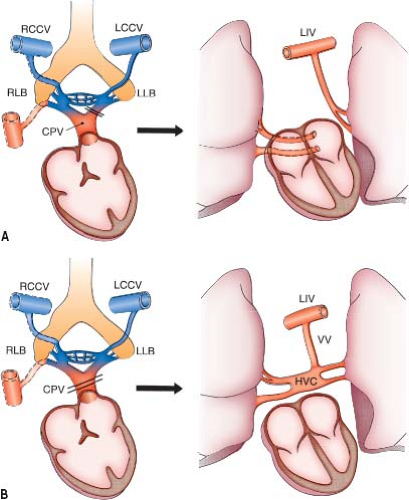
If the common pulmonary vein fails to develop or becomes atretic early in its development, collateral channels for pulmonary venous drainage are available in the form of primitive connections between the splanchnic plexus and the cardinal or umbilicovitelline systems of veins (Fig. 35.2). Any one of these collateral channels may persist and enlarge, resulting in TAPVC. If only the right or left portion of the common pulmonary vein becomes atretic, persistence of the pulmonary venous–systemic venous connections of that side provides the etiologic basis for PAPVC (Fig. 35.2) (5).
TABLE 35.1 Embryologic Classification of Pulmonary Venous Anomalies | |||||
|---|---|---|---|---|---|
|
Late Atresia of the Common Pulmonary Vein after Pulmonary–Systemic Connections are Obliterated
When atresia of the common pulmonary vein occurs late, the collateral venous channels already are obliterated. The resulting anomaly has been termed atresia of the common pulmonary vein. The individual pulmonary veins empty into a blind cul-de-sac that has no direct connection to the LA or to the systemic venous systems (Fig. 35.3A).
Stenosis of the Common Pulmonary Vein
Cor triatriatum is the result of stenosis of the common pulmonary vein (Figs. 35.3B and 35.4). In the usual case, the stenosis occurs late, after collateral venous connections have been lost, or else the severity of the obstruction produced by cor triatriatum is insufficient to stimulate maintenance of the primitive routes of venous drainage. Occasionally, however, cor triatriatum may be associated with anomalous pulmonary venous connection, implying that in such cases, the obstruction was early enough and sufficient to favor persistence of one of the primitive drainage channels such as a levoatriocardinal vein.
Anomalous Incorporation of the Common Pulmonary Vein into the Left Atrium
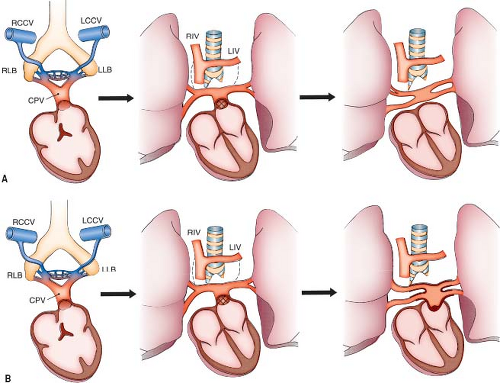
Abnormal numbers of pulmonary veins are the result of imperfect incorporation of the common pulmonary vein into the LA. Incomplete absorption of the common pulmonary vein results in fewer than the normal number of pulmonary veins (1). Rarely, in the mature heart, a common pulmonary vein draining both lungs empties into the LA. More commonly, a single pulmonary vein drains one lung. If more than the usual absorption takes place, there will be an increased number of pulmonary veins.
Stenosis in individual pulmonary veins at their junction with the LA may or may not be a consequence of abnormal incorporation of the common pulmonary vein. One or more of the veins may be affected. Trauma, inflammation, proliferative disorder, or other yet unidentified mechanism(s) may cause stenosis of the individual pulmonary veins.
Early Atresia of the Common Pulmonary Vein while Pulmonary–Systemic Venous Connections are Still Present
Partially Anomalous Pulmonary Venous Connection
PAPVC is the congenital anomaly in which one or more, but not all, of the pulmonary veins are connected to a systemic vein. Winslow is credited with the first description of this anomaly in 1739 (6). By 1942, Brody (7) collected 65 cases from the literature. The advent of surgical correction of cardiac defects necessitated a more critical approach to diagnosis and resulted in the identification and description of hundreds of cases.
Hughes and Rumore (8) found PAPVC in 0.7% of a series of 280 anatomic dissections, and Healy (1) found 0.6% in a series of 801 anatomic dissections. Both figures are higher than those deduced from clinical studies, which implies that some patients with PAPVC are not recognized during life. The most common type of PAPVC is to the right superior vena cava (SVC), and the second most common is to the RA. In Healy’s material, the incidence of those two sites together was 55% of the total number of 86 cases (1).
Our present understanding of the normal connections of the right pulmonary veins (RPV) in sinus venosus defects of the SVC or the right atrial type and in the cases of malposition of the septum primum explains why PAPVD occurs mostly into the right SVC and the RA (see Sections on sinus venosus defects and malposition of the septum primum). In both anomalies, the common pulmonary vein developed normally and is incorporated normally into the LA. The abnormal drainage of the RPVs is due to defects other than abnormal development of the common pulmonary vein. Hence, the actual incidence of partial obliteration of the common pulmonary vein resulting in PAPVC is much lower than thought in the past.
If one considers the cases of sinus venosus defects with the RPVs draining into the SVC or RA, the cases of malposition of the septum primum with drainage of part or all the pulmonary veins into the RA, and cases with partially or totally anomalous pulmonary venous drainage but normal pulmonary venous connections, the incidence of PAPVC to a systemic vein is quite low.
Normal Absorption of the Common Pulmonary Vein with Partially or Totally Abnormal Pulmonary Venous Drainage and Normal Connection of the Pulmonary Veins
Sinus Venosus Defects
Sinus venosus defects allow an atrial-level or a supratrial-level shunt, but they are not atrial septal defects (ASDs). Specifically, these defects do not involve the fossa ovale, septum primum, or septum secundum. They always are associated with drainage of some or all the RPVs into the right SVC or the RA, but the pulmonary veins are normally connected with the LA.
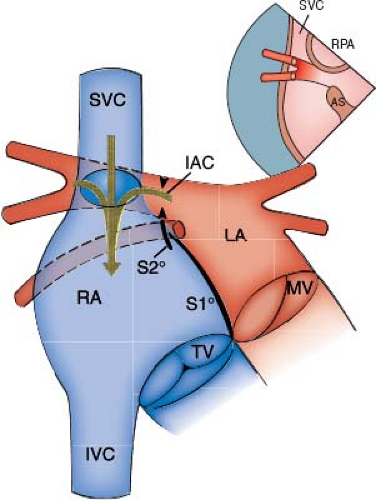
Van Praagh et al.’s study (9) of these defects based on postmortem specimens and echocardiographic studies supports the following conclusions. The true defect is the deficiency of the common wall between the right SVC and the right upper pulmonary vein or the wall between the RA and the right upper and lower pulmonary veins (Fig. 35.5). The result of these deficiencies is the unroofing of
the right upper pulmonary vein and its branches into the right SVC (sinus venosus defects of the SVC type) or the unroofing of the right upper and lower pulmonary veins into the RA (sinus venosus defect of the right atrial type) (9).
the right upper pulmonary vein and its branches into the right SVC (sinus venosus defects of the SVC type) or the unroofing of the right upper and lower pulmonary veins into the RA (sinus venosus defect of the right atrial type) (9).
The interatrial communication is not a defect. It is the left atrial orifice of the unroofed pulmonary veins, as in cases of an unroofed coronary sinus (CS) where the interatrial communication is not a defect; rather, it represents the right atrial orifice of the CS.
When the interatrial communication in sinus venosus defects is viewed from the LA, it is located posterosuperiorly to the upper border of septum primum, exactly in the position of the orifice of the RPVs. In the SVC type of sinus venosus defects, the true defect is much larger than the interatrial communication (the orifice of the right upper pulmonary vein) and occupies all the area where the orifices of the branches of the right upper pulmonary vein are seen to enter the right SVC (Fig. 35.6A). In the right atrial type of
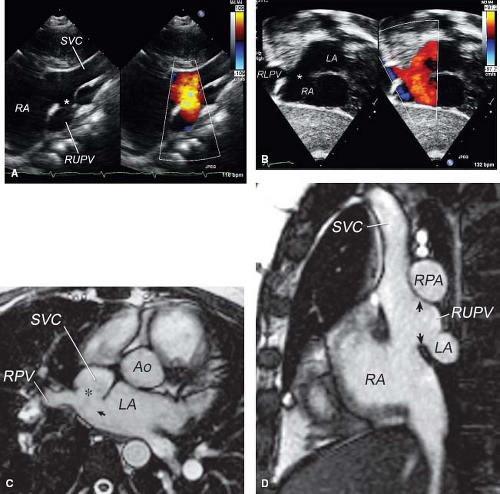
sinus venosus defects, the interatrial communication from the left atrial view is again in the position of the orifice of the RPVs. From the right atrial view, the missing partition between the RPVs and the RA can be imaged clearly by echocardiography (Fig. 35.6B). In both types of sinus venosus defects, the surgeon does not close the interatrial communication. Instead, the operation aims to restore the original function of the interatrial communication as the left atrial orifice of the RPVs (9), which can be accomplished by “reroofing” the pulmonary veins, that is, by patching the missing wall between the SVC and the pulmonary veins or between the RA and the pulmonary veins. The true nature of the interatrial communication, the left atrial orifice of the RPV(s), is thus restored.
sinus venosus defects, the interatrial communication from the left atrial view is again in the position of the orifice of the RPVs. From the right atrial view, the missing partition between the RPVs and the RA can be imaged clearly by echocardiography (Fig. 35.6B). In both types of sinus venosus defects, the surgeon does not close the interatrial communication. Instead, the operation aims to restore the original function of the interatrial communication as the left atrial orifice of the RPVs (9), which can be accomplished by “reroofing” the pulmonary veins, that is, by patching the missing wall between the SVC and the pulmonary veins or between the RA and the pulmonary veins. The true nature of the interatrial communication, the left atrial orifice of the RPV(s), is thus restored.
Malposition of Septum Primum
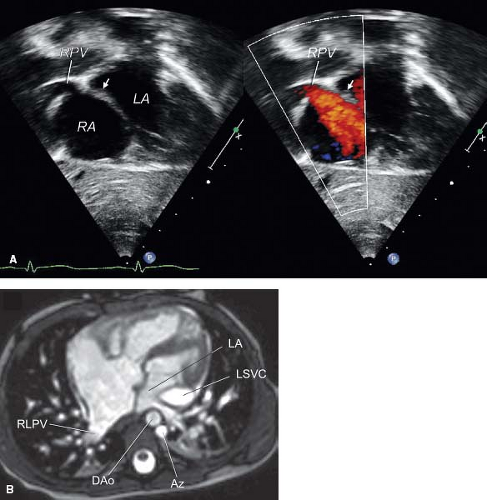
Edwards (2) and Moller et al. (10) observed that, when the septum secundum is absent (usually in cases of visceral heterotaxy with polysplenia), septum primum may be displaced toward the anatomic LA (left-sided or right-sided, depending on the type of atrial situs). This displacement of the upper border of the septum primum will result in the incorporation of some or even all the pulmonary veins into the morphologic RA. Although this malformation differs from sinus venosus defects, here also the normally connected pulmonary veins will drain into the RA. The two-dimensional (2-D) echocardiograms of such patients will show the change in the plane of the septum primum and the normal connection of the pulmonary veins in the back wall of the atrium (Fig. 35.7). The displaced septum primum may not reach the posterior wall of the LA. In such cases, there will be a small interatrial communication that is not an ASD or a patent foramen ovale; rather, it is a septum primum malposition defect. If the septum primum has some fenestrations (foramina secunda), multiple small interatrial communications will be present. Finally, in cases where septum primum fuses with the posterior left atrial wall, there is no interatrial communication. Supporting evidence for the conclusion that the pulmonary veins are connected normally to the atrial wall is that they are located between the right and left SVCs (when two SVCs are present). This is the normal location for the atrial connection of the common pulmonary vein (i.e., between the two horns of the sinus venosus just above the CS). The absence of septum secundum makes it possible to visualize the attachments of septum primum from the right atrial side in heart specimens, a unique finding of this malformation. The echocardiographic appearance of the displaced mobile upper border of the septum primum helps to establish the diagnosis in vivo.
The essential anatomic elements that make possible the malposition of the septum primum toward the anatomic LA include
absence of septum secundum and a well-developed septum primum. Those two elements often are present in patients with visceral heterotaxy and polysplenia. Patients with asplenia, however, seldom have a well-developed septum primum that could become malpositioned. Instead, they often exhibit TAPVC to a systemic vein above or below the diaphragm.
absence of septum secundum and a well-developed septum primum. Those two elements often are present in patients with visceral heterotaxy and polysplenia. Patients with asplenia, however, seldom have a well-developed septum primum that could become malpositioned. Instead, they often exhibit TAPVC to a systemic vein above or below the diaphragm.
Partially Anomalous Pulmonary Venous Connections
Anatomy
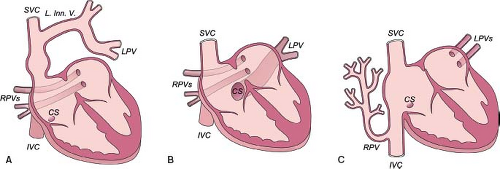
PAPVCs exhibit a wide anatomic spectrum. Almost every conceivable connection between the pulmonary veins, on the one hand, and the various systemic venous tributaries, on the other hand, has been reported (Fig. 35.8). Left-sided pulmonary veins usually connect anomalously to derivatives of the left cardinal system (i.e., the CS and the left innominate vein [LIV]). Anomalous connections of the RPVs usually are to derivatives of the right cardinal system (i.e., the SVC or inferior vena cava [IVC]). The embryologic splanchnic plexus is a midline structure, thus explaining the developmental possibility for crossed drainage of left-sided pulmonary veins to derivatives of the right cardinal system and vice versa. Excluding the cases previously considered PAPVC to the right SVC and to the RA (now considered under sinus venosus defect and malposition of the septum primum), the most common type of PAPVC is of the left pulmonary veins to the LIV. The second most common types are anomalous connections of pulmonary veins from the right lung to the IVC.
Right Pulmonary Veins to Superior Vena Cava
As outlined in the section on sinus venosus defects, the RPVs may drain into the right SVC or into the RA without being abnormally connected with them. The upper lobe drains by one large or two or three smaller veins into the SVC below the azygos vein. The multiple orifices indicate that the unroofing of the right upper pulmonary veins extends into their branches. The vein from the right lower lobe usually enters the LA. The lower part of the SVC, between the azygos vein and RA, is dilated. An interatrial communication (the left atrial orifice of the unroofed pulmonary veins) usually is present. Occasionally, a secundum ASD also is present. Rarely an ostium primum ASD also exists. When the orifice of the right upper pulmonary vein is atretic, the atrial septum is intact. Persistence of a left SVC is an occasional accompanying finding.
Anomalous connection of a single pulmonary vein to the right SVC as an isolated lesion or in combination with a sinus venosus defect is possible. The anomalously connected pulmonary vein in such a case originates from the right upper lobe and enters the right SVC either through the azygos vein or cranial to the insertion of the azygos vein to the SVC.
Gross examination of the heart reveals features common to all cases regardless of the specific site of anomalous connection. These include mild to moderate dilation and hypertrophy of the RA and right ventricle and dilation of the pulmonary artery. The left-sided chambers are normal. Specific anatomic features vary according to the site of connection.
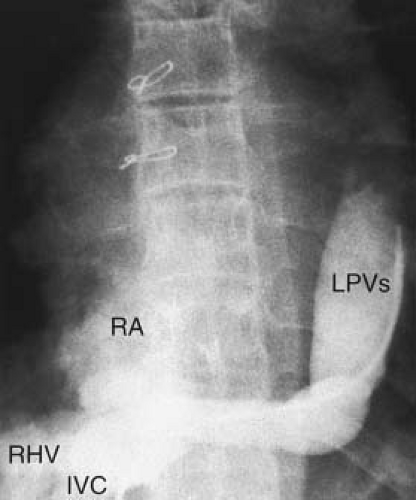
Right Pulmonary Veins to Inferior Vena Cava
All the RPVs or occasionally the veins draining the right middle and right lower lobes enter the IVC either just above or below the diaphragm (Fig. 35.9). The normal pulmonary venous pattern of the right lung is altered in this condition, resulting in a “fir tree” configuration. The atrial septum is usually intact. This malformation, termed the scimitar syndrome by Neill et al. (5), frequently is associated with other anomalies, including hypoplasia of the right lung, anomalies of the bronchial system, horseshoe lung, secondary dextrocardia, hypoplasia of the right pulmonary artery, anomalous arterial connection to the right lung from the aorta, and pulmonary sequestration (11). Neill et al. (5) considered this a more primitive anomaly compared with other instances of PAPVC and suggested that it represents an anomaly of development of the right lung.
Left Pulmonary Veins to Inferior Vena Cava
Rarely, some or all of the left pulmonary veins can drain into the IVC. Juraszek et al. observed a case in which all the left pulmonary veins connected to the IVC below the diaphragm in association with an ostium secundum defect (Fig. 35.10). There was no additional heart defect, and both lungs were normal (12). Others also have reported cases of left-sided scimitar syndrome (13,14).
Left Pulmonary Veins to Left Innominate Vein
The LIV is the usual site of anomalous connection of the left pulmonary veins (see Fig. 35.8A). The veins from the left upper lobe or from the whole left lung connect to the LIV via a persistent early embryonic pathway, which, because of its orientation, has been termed a vertical vein. An interatrial communication in the form of a secundum ASD or a patent foramen ovale is common.
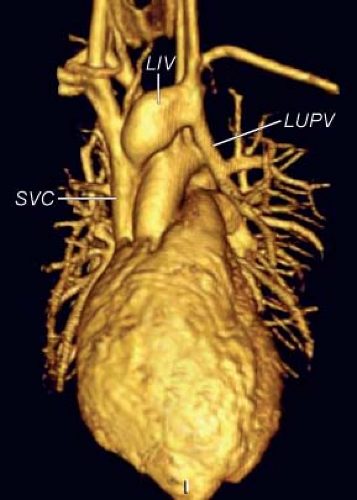
The connecting vein between the left pulmonary veins and the LIV also has been called a persistent left superior vena cava (LSVC). This term is incorrect both embryologically and anatomically. Embryologically, the vertical vein represents a persistent early embryonic connection between the splanchnic plexus of the lung buds and the cardinal veins. Anatomically, it is positioned more posteriorly than the LSVC, which is located immediately behind the left atrial appendage. An LSVC connects either with the CS or with the LA when the CS is unroofed. When a left pulmonary vein drains into the LSVC, the LSVC also should connect with the CS or with the LA. In a rare case (1), the left upper pulmonary vein (LUPV) joined the left superior intercostal vein, which in turn joined the LSVC. The confluence of those three veins entered the LIV. The proximal segment of the LSVC was atretic (ligament of Marshall) but present. To avoid inadvertent occlusion of pulmonary venous drainage, it is important that the differential diagnosis between a vertical vein and persistent LSVC be made before performance of any surgical or transcatheter intervention.
Other Sites of Partially Anomalous Pulmonary Venous Connection
Uncommon sites of PAPVC of the left pulmonary veins are to the CS (see Fig. 35.8B), IVC, right SVC, left subclavian vein, and azygos vein. In rare cases, veins of both lungs connect anomalously to systemic veins, while some of the pulmonary veins connect normally to the LA. Functionally, these patients resemble patients with TAPVC.
Physiology
The fundamental physiologic disturbance of PAPVC is similar to that in ASD, that is, increased pulmonary blood flow (PBF) as a consequence of recirculation of oxygenated blood through the lungs. The factors that determine the hemodynamic state include the number of anomalously connected veins, the cross-sectional area of the anomalously draining pulmonary vascular bed, the site of the anomalous connections, the presence or absence of an ASD, and the size of the ASD.
Partially Anomalous Pulmonary Venous Connection with Intact Atrial Septum
When the atrial septum is intact, factors that determine the proportion of blood that drains through the anomalously connected veins include the number of veins that are anomalously connected and the amount of lung tissue involved, the relative resistance of the vascular beds normally and anomalously connected, compliance of the respective atria into which the normally and anomalously connected veins empty, and the presence and degree of obstruction to pulmonary arterial blood flow.
When a single pulmonary vein is anomalously connected, the anomalously draining blood flow is about 20% to 25% of the total PBF (6). It is of such slight hemodynamic significance that the lesion is rarely recognized clinically. Hughes and Rumore (8) found neither dilation nor hypertrophy of the right heart in two patients with a single-lobe PAPVC who died at middle age of rheumatic heart disease. When all but one of the pulmonary veins drain anomalously, the anomalously draining blood approximates 80% of the PBF. The physiology and clinical presentation of these patients are comparable to those of the patient with TAPVC.
When the veins of one lung drain anomalously, the factors of relative pulmonary vascular resistance and relative receiving chamber compliance modify the relative blood flows. In one study of patients with PAPVC of the RPVs to the IVC with intact atrial septum (scimitar syndrome), calculated PBF to the anomalously connected vein was 24% to 32% of the PBF (15). This low flow is related to abnormalities of the right lung parenchyma and the frequently associated anomalies of arterial supply that are seen in the scimitar syndrome (17). On the other hand, when anomalous connection of one lung is the sole abnormality, the anomalously draining blood flow approximates 66% of the PBF as a result of the greater compliance of the RA, to which the anomalous veins drain, and the lesser compliance of the LA, the chamber receiving the normally draining blood. Thus, in patients in whom partially anomalous venous connection is the sole abnormality, the right atrial pressure is usually lower than left atrial pressure. As long as pulmonary vascular resistance remains equal in both lungs and there is no pulmonary arterial stenosis, blood flow is greater in the anomalously connected lung.
The lobe or lobes drained by the anomalously connecting pulmonary vein also affect the magnitude of the left-to-right shunt. In the upright position at rest, PBF is distributed preferentially to the middle and lower lobes. In supine position and during exercise, PBF is redistributed to the upper lobes. Hence, the magnitude of the left-to-right shunt in a patient with PAPVC from one of the upper lobes may vary according to body position and level of activity.
Partially Anomalous Pulmonary Venous Connection with Atrial Septal Defect
When the ASD is small, the hemodynamic state closely resembles that of PAPVC with an intact septum. On the other hand, a large ASD significantly influences the hemodynamic picture. It is pertinent to review briefly the physiology of uncomplicated ASD, in particular, the preferential shunting that occurs in this anomaly.
Using indicator dye dilution techniques, Burchell et al. (16) evaluated the relative contribution of each lung to the left-to-right shunt in patients with ASD. The average ratio of PBF to systemic blood flow (SBF) was 3.5:1. The proportion of blood from the right lung that was shunted left to right averaged 84%, whereas the proportion of blood from the left lung that was shunted averaged 54%. Thus, blood from both lungs drained anomalously, but the right lung contributed more than the left lung to left-to-right shunt. This preferential shunting from the right lung in ASD has been shown experimentally to result from the proximity of the right pulmonary venous orifices to the ASD.
When PAPVC and ASD coexist, the hemodynamic picture may be similar to that of uncomplicated ASD. The left-to-right shunt may be large. This shunt is the result of anomalous drainage of most of the blood from the anomalously connected lung and of anomalous drainage of half or more of the blood from the normally connected lung via the ASD (16).
A small right-to-left shunt from the SVC (both systemic venous blood and anomalously draining pulmonary venous blood) is usual in the presence of a sinus venosus defect. Likewise, a small right-to-left shunt from the IVC is commonly seen in a secundum ASD. Pulmonary vascular disease has been reported in older patients with uncomplicated PAPVC (17). Although pulmonary hypertension is thought to be rare, its prevalence is unknown.
Clinical Features
The anomalous connection of one pulmonary vein usually is not apparent clinically. If all but one of the veins connect anomalously (subtotal TAPVC), the clinical features mimic those of TAPVC. The following features occur when the veins of one lung connect anomalously. Symptoms are uncommon in childhood, but some dyspnea may occur on exertion. Cyanosis is unusual during childhood, even though a small right-to-left shunt may exist, depending on the location of the PAPVC. The frequency of patients presenting with cyanosis increases during the third and fourth decades as a result of changes in the pulmonary vascular bed, pulmonary hypertension, and increasing right-to-left shunt.
The clinical presentation of patients with the scimitar syndrome varies widely. Dupuis et al. (18) reported on 25 infants with the
scimitar syndrome, and Gao et al. (11) reviewed the data of 13 such patients. All but two patients presented with severe symptoms in early infancy. Pulmonary hypertension was common and was secondary to a combination of conditions, including arterial blood supply from the descending aorta, stenosis of the anomalously connecting RPVs, pulmonary parenchymal abnormalities, and associated obstructive anomalies of the left heart and aorta. Hypoplasia of the right lung with secondary dextrocardia was common. In contrast, in a study of 122 patients with scimitar syndrome who presented later in life (the adult form of scimitar syndrome), symptoms were rare, the left-to-right shunt was <50% in 100 of the 122 patients, the pulmonary artery pressure was normal in 94 and mildly elevated in 28 patients, and the clinical outcome was good in most of these patients (19).
scimitar syndrome, and Gao et al. (11) reviewed the data of 13 such patients. All but two patients presented with severe symptoms in early infancy. Pulmonary hypertension was common and was secondary to a combination of conditions, including arterial blood supply from the descending aorta, stenosis of the anomalously connecting RPVs, pulmonary parenchymal abnormalities, and associated obstructive anomalies of the left heart and aorta. Hypoplasia of the right lung with secondary dextrocardia was common. In contrast, in a study of 122 patients with scimitar syndrome who presented later in life (the adult form of scimitar syndrome), symptoms were rare, the left-to-right shunt was <50% in 100 of the 122 patients, the pulmonary artery pressure was normal in 94 and mildly elevated in 28 patients, and the clinical outcome was good in most of these patients (19).
In the presence of an associated ASD, the physical findings are identical to those noted in uncomplicated ASD. When the atrial septum is intact, splitting of the second sound is not marked, and there is normal variation of splitting with respiration. A pulmonary outflow murmur is usually present, and a diastolic tricuspid flow murmur may be present.
Associated Cardiac Defects and Syndromes
PAPVC or drainage has been associated with other congenital cardiac defects and syndromes. Most notably, patients with visceral heterotaxy and polysplenia have a high incidence of PAPVD secondary to malposition of the septum primum, and patients with asplenia have a high incidence of TAPVC. Recent reports described a significant incidence of PAPVC in association with Turner and Noonan syndromes. A prospective evaluation of 21 patients with Turner syndrome by Moore et al. (20) showed three cases of PAPVC with intact atrial septum (14.3%). Of these 21 patients, 12 had the 45 XO karyotype. PAPVC was seen only in this latter population (incidence of 25%) but did not occur in the nine patients who had Turner syndrome of the mosaic type. Additionally, van Wassenaer et al. (21) reported the association of PAPVC with intact atrial septum in three patients with Turner syndrome. Hence, the possibility of PAPVC should be considered in any patient with either Turner or Noonan syndrome.
A rare but clinically important association is that of anomalous pulmonary venous connections with tetralogy of Fallot. A review of 1,183 patients with tetralogy of Fallot (22) described seven patients with anomalous pulmonary venous connections (0.6%). PAPVC was present in four patients, whereas the other three patients had TAPVC.
Diagnostic Features
Electrocardiographic Features
The electrocardiographic (ECG) findings are comparable to those seen in uncomplicated ASD. The rR′ pattern and the rSR′ pattern are most commonly seen; the ECG is occasionally normal. Peaked P waves and right ventricular hypertrophy of the systolic overload pattern occur in older patients exhibiting pulmonary hypertension. A frontal P-wave axis of ≤15 (inverted P wave in lead III) has been described with sinus venosus defect. The ECG is often normal in the patient with intact atrial septum.
Radiologic Features
The routine chest radiogram reflects the increased PBF and right ventricular dilation. Additionally, there may be distinctive features dependent on the site of anomalous connection.
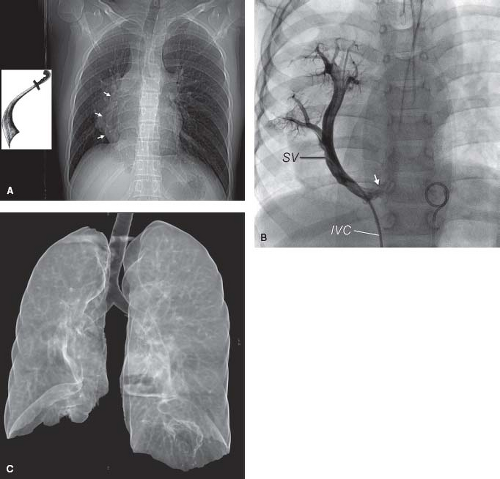
The patient with anomalous connection of the RPVs to the IVC has a crescent-like shadow in the right lower lung field (see Fig. 35.9B). This characteristic shadow prompted Neill et al. (5) to coin the term scimitar syndrome for this group of patients. Hypoplasia of the right lung (see Fig. 35.9C) and chest, mesocardia or dextrocardia, and pulmonary parenchymal abnormalities also may be seen on the chest radiogram when drainage is into the IVC.
When the SVC is the site of the anomalous connection, dilation of the lower portion of the SVC is often recognized just above the right atrial contour or as a double density just inside the upper atrial border. When the anomalous connection is to the azygos vein, this structure is enlarged and can be recognized on the chest radiogram as a rounded bulge in the right superior mediastinum at the right cardiac border.
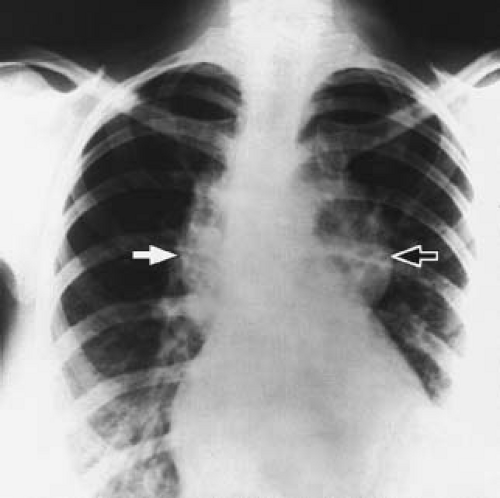
When the LIV is the site of connection of the left pulmonary veins, the chest radiogram shows a prominent supracardiac shadow composed of the vertical vein on the left, the innominate vein above, and the SVC at the right (Fig. 35.11). These structures are the same ones that account for the characteristic “snowman” appearance when there is a TAPVC to the innominate vein, but the enlargement is not as prominent.
Echocardiographic Features
The key to accurate echocardiographic diagnosis of PAPVC and PAPVD is detailed comprehensive imaging of all cardiovascular structures. The individual pulmonary veins should be examined in every patient, particularly at the time of the first echocardiographic
evaluation. The size and course of the individual pulmonary veins must be determined both by 2-D imaging and by color Doppler flow mapping. The connection and drainage of the right upper and right lower and the left upper and left lower pulmonary veins with the LA should be demonstrated unequivocally. This can be achieved from the subcostal, apical, parasternal, and suprasternal notch windows. The subcostal window is ideal for evaluating the pulmonary veins in infants and young patients with good subcostal acoustic windows. The parasternal, subclavicular, and suprasternal windows are used in older patients. Transesophageal echocardiography is useful in patients with suboptimal transthoracic acoustic windows. Magnetic resonance imaging (MRI) is a preferred noninvasive alternative to transesophageal echocardiography (see later discussion). The presence of right ventricular volume overload, manifested as right ventricular enlargement with flattening of the ventricular septum during diastole, is a useful clue for the presence of a left-to-right shunt but is not specific for PAPVC. The finding of right ventricular volume overload in the absence of an ASD should prompt a careful search for PAPVC.
evaluation. The size and course of the individual pulmonary veins must be determined both by 2-D imaging and by color Doppler flow mapping. The connection and drainage of the right upper and right lower and the left upper and left lower pulmonary veins with the LA should be demonstrated unequivocally. This can be achieved from the subcostal, apical, parasternal, and suprasternal notch windows. The subcostal window is ideal for evaluating the pulmonary veins in infants and young patients with good subcostal acoustic windows. The parasternal, subclavicular, and suprasternal windows are used in older patients. Transesophageal echocardiography is useful in patients with suboptimal transthoracic acoustic windows. Magnetic resonance imaging (MRI) is a preferred noninvasive alternative to transesophageal echocardiography (see later discussion). The presence of right ventricular volume overload, manifested as right ventricular enlargement with flattening of the ventricular septum during diastole, is a useful clue for the presence of a left-to-right shunt but is not specific for PAPVC. The finding of right ventricular volume overload in the absence of an ASD should prompt a careful search for PAPVC.
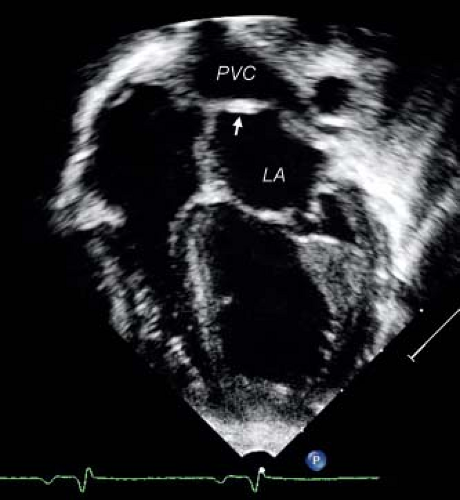
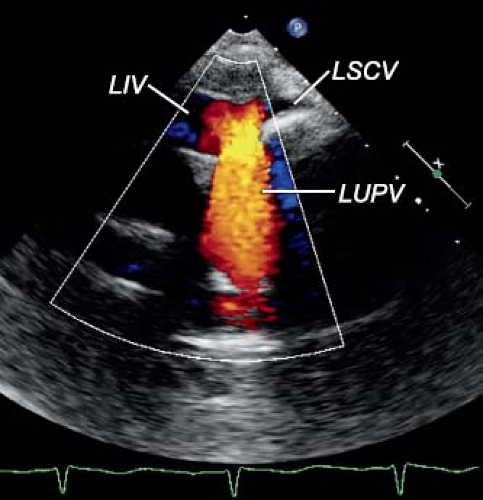
The echocardiographic diagnosis of PAPVC is based on the demonstration of a connection between one or more of the pulmonary veins with a systemic vein. Typically, the systemic vein distal to the connection of the pulmonary vein is dilated, reflecting the increased flow. Anomalous connection of pulmonary veins to the SVC is best imaged from the suprasternal notch or high parasternal long axis of the SVC. Similarly, high parasternal short- and long-axis views allow visualization of the anomalous connection of the pulmonary veins to the LIV (Fig. 35.12). The diagnosis can be made from the subcostal window in infants and young children. Enlargement of the LIV and the SVC associated with evidence of increased flow by Doppler are clues to the diagnosis.
The anomalous pulmonary venous connection to the CS is best imaged by scanning the posterior left atrioventricular (AV) groove from the subcostal, apical, and parasternal windows. The dilated CS in this condition should be distinguished from the more common persistent LSVC.
Imaging of the anomalous connection of one or more of the RPVs with the IVC establishes the diagnosis of scimitar syndrome. The subcostal window is most useful in this condition. The presence of mesocardia or dextrocardia in 70% of patients and a smaller-caliber right pulmonary artery compared with the left pulmonary artery are useful clues. Other findings include blunting of the right border of the LA when both RPVs do not connect with the LA and demonstration of aortopulmonary collaterals by color Doppler flow mapping.
Sinus venosus defect of the inferior type can be a challenging diagnosis to make by echocardiography, particularly in distinguishing this entity from a posteriorly located secundum ASD. Banka et al. (23) demonstrated in a group of 45 patients with inferior-type sinus venosus defect that the rate of accurate preoperative diagnosis was only 36% and that patients with incorrect diagnoses had worse technical outcome scores postoperatively compared with those with the correct preoperative diagnosis (23).
Magnetic Resonance Imaging
Cardiac MRI is suited ideally for evaluation of pulmonary venous anomalies (Fig. 35.13). The wide field of view, excellent spatial orientation, and its inherent three-dimensional (3-D) nature allow unambiguous delineation of the course, connections, and drainage of the pulmonary veins independent of body size and acoustic windows. Also, the ability to depict noncardiovascular structures such as lung parenchyma, airways, bones, and soft tissue offer an advantage over echocardiography and angiocardiography. Another advantage of MRI is its ability to measure the hemodynamic consequences of anomalous pulmonary venous connections and drainage, including the pulmonary-to-systemic flow ratio and right ventricular size and function.
The anatomy of the pulmonary veins can be evaluated by a series of T1-weighted spin echo images in the axial (transverse), coronal, or oblique planes. This sequence offers excellent spatial resolution but provides only one image in each location. The use of ECG-gated cine MRI sequence allows rapid acquisition of multiple contiguous cine MRIs slabs that cover the chest. Cine steady-state free precession MRI clearly depicts the size, course, and drainage of the pulmonary veins and their relations to neighboring structures. Turbulent flow is seen clearly by this sequence. The advent of magnetic resonance angiography (MRA) added a powerful tool that allows fast 3-D imaging of cardiovascular structures (Figs. 35.13 and 35.14). Phase-contrast cine MRI allows quantification of blood flow through the pulmonary veins, determination of systemic and pulmonary flow (Qp:Qs), and flow to the left and right lungs. Experience with MRI and MRA in the evaluation of anomalous pulmonary venous connections indicates that it is superior to echocardiography and angiocardiography (24,25).
Cardiac Catheterization
Cardiac catheterization usually is not necessary for the diagnosis of PAPVC, its differentiation from secundum ASD, or estimation of the magnitude of the left-to-right shunt. Clinical features, echocardiography, and MRI provide sufficient anatomic and hemodynamic preoperative data. Cardiac catheterization is still useful in selected cases, especially if pulmonary hypertension is suspected. Interventional catheterization is indicated to occlude aortopulmonary collaterals in scimitar syndrome.
When cardiac catheterization is performed, frequent oximetry sampling usually allows identification of anomalous pulmonary venous connection to the CS, to the azygos vein, and to the SVC. Oximetry is usually of little value when the anomalous connection is to the IVC because blood flow through the right lung is diminished and also because of the contribution of highly oxygenated blood to the IVC by the renal veins. Inability to pass the catheter from the RA to the LA or a difference between the right atrial pressure and the pulmonary wedge pressure is suggestive of PAPVC with intact atrial septum.
Selective angiography is of limited value if the anomalously draining veins enter the RA or close to it. When the anomalous connections are to more peripheral vessels, however, angiography usually can define the site of anomalous connection, for example, PAPVC to the IVC, SVC, LIV, and azygos vein. Performing selective pulmonary arteriography and watching the levophase for pulmonary venous return may image the connections. If the anomalous connection is entered, direct injection of radiographic contrast dye will delineate the anatomy.
Differential Diagnosis
The distinction between ASD and PAPVC by clinical evaluation is difficult to make but is academic. The capabilities of today’s noninvasive imaging techniques (echo and MRI) allow accurate preoperative diagnosis. Ostium primum ASD and single atrium also result in left-to-right shunt at the atrial level; these may be differentiated on the basis of their characteristic ECG pattern (i.e., counterclockwise frontal vector loop and left-axis deviation). TAPVC presents a strikingly different clinical picture, with severe pulmonary congestion and cardiac failure, usually leading to death prior to 6 months of age if not treated. Cyanosis is an additional distinguishing feature.
Treatment
Medical Management
When heart failure occurs, either as a result of increased PBF or as a consequence of pulmonary vascular disease, the usual measures are indicated. When pulmonary parenchymal anomalies are associated, as in anomalous connection to the IVC, medical measures may be of temporary benefit. The only definitive therapy in PAPVC, however, is surgical correction.