Chapter 22 Angioplasty and Stenting for Aortoiliac Disease
Technique and Results
History of Endoluminal Treatment
The concept of endovascular therapy of atherosclerotic occlusive disease was introduced in the 1960s when Dotter performed the first transluminal angioplasty in a patient with ischemic extremities.1 His novel technique, however, could not reliably maintain luminal patency and did not receive wide acceptance as a treatment of vascular occlusive lesions. Subsequent device modifications led to the development of an angioplasty balloon composed of latex material. The clinical success of this new construct was limited, in part because of its improved compliance, and could not fully dilate a calcified lesion. The introduction of an angioplasty balloon made of polyvinyl chloride in 1976 by Gruntzig and Hopff,2 followed by rigid balloons composed of polyethylene and polyethylene terephthalate, marked a significant advance in endovascular therapy. The latter angioplasty balloons displayed a low compliance and high radial force in a fully inflated condition. As the technology and techniques of endovascular therapy continued to improve as well as become more widely accepted as outcomes data became available, transluminal balloon angioplasty evolved to an important modality in the treatment of peripheral vascular disease.
Despite numerous studies demonstrating the short-term clinical success of transluminal angioplasty, it has several limitations, in part because of the architectural variation of atherosclerotic lesions. Early restenosis or occlusion following angioplasty can occur because of either elastic recoil of the arterial wall or intimal dissection.3,4 Vessels that are heavily calcified, completely occluded, or that contain ulcerated plaques might not be amenable to balloon angioplasty. Moreover, residual luminal irregularities following angioplasty occasionally trigger mural thrombus formation, leading to thrombotic occlusion.5,6 Late restenosis following angioplasty can occur as a result of intimal hyperplasia or atherosclerotic progression.7–9
In an effort to deal with these limitations of transluminal angioplasty, researchers proposed the concept of intravascular stents as a means of maintaining vessel patency against the restenotic process, as well as improving the clinical outcome of balloon angioplasty. Since Dotter first reported his successful deployment of intravascular stents in canine femoral and popliteal arteries in 1969,10 a variety of stent devices composed of various materials have been introduced.11–14 Intravascular stenting is recognized as a potentially effective modality in overcoming elastic recoil of the arterial wall, stabilizing intimal dissection at the site of angioplasty, and maintaining the luminal patency of arteries with calcified and eccentric atherosclerotic plaques.
Classification of Aortoiliac Occlussive Disease
The 2007 Trans-Atlantic Inter-Society Consensus (TASC II) guidelines were developed to assist physicians in the prevention and management of peripheral arterial disease.15 The classification of inflow of aortoiliac lesions was updated in 2007 to reflect the advances in endovascular technology. General guidelines included in the TASC II document state that lesions classified as TASC A (Table 22-1) should be treated preferentially with endovascular techniques, whereas lesions classified as TASC D should be treated preferentially with open surgery. Lesions classified as TASC B or C have less clear recommendations. Good surgical judgment including surgical experience, available technology, and patient overall health needs to be considered when deciding on the appropriate initial treatment plan.
TABLE 22-1 TransAtlantic Inter-Society Consensus II: Morphologic Stratification of Iliac Lesions
| Type | Definition | Treatment Choice |
|---|---|---|
| A | Unilateral or bilateral stenoses of CIA | Endovascular |
| Unilateral or bilateral single short (<3 cm) stenosis of EIA | ||
| B | Short (≤3 cm) stenosis of infrarenal aorta | Endovascular preferred |
| Unilateral CIA occlusion | ||
| Single or multiple stenosis totaling 3-10 cm involving the EIA not extending into the CFA | ||
| Unilateral EIA occlusion not involving the origins of internal iliac or CFA | ||
| C | Bilateral CIA occlusionsBilateral EIA stenoses 3-10 cm long, not extending into CFA for good-risk patients | Open surgery preferred |
| Unilateral EIA stenosis extending into the CFA | ||
| Unilateral EIA occlusion that involves the origins of the internal iliac and/or CFA | ||
| Heavily calcified unilateral EIA occlusion with or without involvement of origins if internal iliac and/or CFA | ||
| D | Infrarenal aortoiliac occlusion | Open surgery |
| Diffuse disease involving the aorta and both iliac arteries requiring treatment | ||
| Diffuse multiple stenoses involving the unilateral CIA, EIA, and CFA | ||
| Unilateral occlusions of both CIA and EIA | ||
| Bilateral occlusions of EIA | ||
| Iliac stenosis in patients with AAA requiring treatment and not amendable to endograft placement or other lesions requiring open aorta or iliac surgery |
CIA, Common iliac artery; EIA, external iliac artery; CFA, common femoral artery; AAA, abdominal aortic aneurysm.
General Principles of Endoluminal Stents
Intravascular stents can be separated into three distinct types: self-expanding stents, balloon-expandable stents, and covered stents (Table 22-2). Within each of these categories, there are numerous stents with different structural materials, deployment devices, and biocompatible characteristics.11,12,16–19 In addition, there are many stents undergoing clinical trials. However, the Wallstent (Schneider, Minneapolis, Minn.), the Palmaz stent (Cordis, Warren, N.J.), the Medtronic Vascular Complete SE Vascular Stent System (Medtronic Vascular, Santa Rosa, Calif.), the Express LD Iliac Premounted Stent System (Boston Scientific Corp, Natick, Mass.), the E-LUMINEXX Vascular Stent (Bard Peripheral Vascular, Tempe, Ariz.), the Zilver Vascular Stent (Cook, Bloomington, Ind.), the S.M.A.R.T. Nitinol Stent System (Cordis, Miami Lakes, Fla.), the Viabahn endoprosthesis (W.L. Gore, Flagstaff, Ariz.) have been approved by the U.S. Food and Drug Administration (FDA) specifically for iliac artery placement.
TABLE 22-2 Characteristics of Stents
| Self-Expanding | Balloon-Expandable | Covered Stent |
|---|---|---|
| Flexible | Rigid | Flexible |
| Low radial force | High radial force | Low radial force |
| Longer lengths | More effective at short lengths | Longest lengths available |
| Covering sheath in delivery system | Premounted on a balloon | Sheath or balloon premounted |
| Shortening is variable | Some shortening | Minimal shortening |
| Nitinol frame | Steel frame | Nitinol or steel frame |
| Radiopacity is low | Radiopacity is moderate | Radiopacity is moderate |
| Less accurate placement | Accurate placement | Variable accuracy |
Self-Expanding Stents
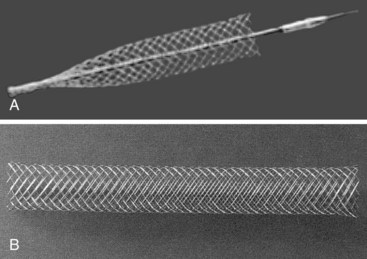
Self-expanding stents are generally manufactured so that the stents are constrained within a delivery sheath. The prosthesis is placed intravascularly through an introducer sheath that is advanced over a guidewire for deployment. Deployment is achieved by withdrawing the constraining sheath while keeping the stent in position, thus permitting the stent to self-expand and anchor to the vessel lumen (Figure 22-1). Stents in this category are generally noted for their ease of deployment and high degree of flexibility meandering around the curves typically found in an iliac artery. These characteristics are advantageous because they tend to be more forgiving if they are not placed exactly accurately. Compared with balloon-expandable stents, however, they may have lower hoop strength or radial force, which is the resistance to radial compression. These stents require oversizing by 2 to 3 mm to maintain outward radial force after deployment. Most delivery systems of self-expanding stents have a smaller diameter compared with those of balloon-expandable stents. Besides, the Wallstent and the Gianturco-Z stent (Cook, Bloomington, Ind.) are available.
Another type of self-expanding stent is the nitinol stent, which was first introduced by Dotter and colleagues in 1983.20 Nitinol is a nickel-titanium alloy that has a unique temperature-associated memory property. The nitinol wires can be shaped into a coil spring configuration to serve as a stent when heated to 500°C. As it cools down to 0°C, the nitinol coil straightens into a linear alignment that can be constrained into a delivery catheter for stent deployment. The exposure to warm body temperature after deployment causes the nitinol stent to resume its original coil spring shape. Several clinical studies have evaluated the application of these stents in vascular occlusive lesions.20–23 Common examples of nitinol stents include the Symphony stent (Boston Scientific Vascular, Watertown, Mass.), Cragg stent (Mintech, France, Elsenfeld, Germany), S.M.A.R.T. stent (Cordis, Miami, Fla.), and Memotherm stent (Bard/Angiomed, Karlsruhe, Germany).
Balloon-Expandable Stents
The balloon-expandable stent is first mounted and compressed onto an angioplasty balloon. The original stents were all operator mounted; newer stents are premounted by the manufacturer. Once the stent is positioned intraluminally, it is deployed by inflating the balloon to expand the stent (Figure 22-2). Unlike a self-expanding stent, the balloon-expandable stent can be expanded further by a larger angioplasty balloon catheter beyond its predetermined diameter. Most balloon-expandable stents are characterized by excellent hoop strength owing to intrinsic stent rigidity once deployed, which can be advantageous in treating stenotic vessels containing calcified plaques. The rigid property of such a stent, however, can create a technical dilemma when treating lesions in tortuous vessels. Moreover, it makes a contralateral approach for iliac artery stenting a challenge, although newer stents are more trackable and flexible. Commonly used examples of balloon-expandable stents include the Palmaz stent (Johnson and Johnson Interventional Systems, Warren, N.J.), Strecker stent (Boston Scientific Vascular, Watertown, Mass.), Gianturco-Roubin stent (Cook, Bloomington, Ind.), Ave stent (Arterial Vascular Engineering, Santa Rosa, Calif.), Absolute Stent (Medtronic, San Diego, Calif.), and Intrastent (Intratherapeutics, Minneapolis, Minn).
Covered Stents
The first covered stent approved by the FDA for the use in the iliac system is the Gore Viabahn (Gore, Flagstaf, Ariz.). It is a flexible Nitinol stent lined with expanded polytetrafluoroethylene. The Nitinol flexibility, as in self-expanding stents, allows it to conform to the vessel shape closely. In addition, it has a heparin-bioactive surface providing a thromboresistent surface. It is important to conduct predeployment percutaneous transluminal angioplasty (PTA) of the affected area with subsequent coverage of the entire PTA-treated area with the covered stent. This stent is deployed by untwisting the screw-connector at the base of the deployment knob and slowly pulling the knob away from the adapter. In the Gore Viabahn Endoprosthesis Feasibility Study, 45 limbs in 42 subjects were treated for iliac artery occlusive disease.24 This was the first-generation endoprosthesis without the heparin bioactive surface. There was a 12-month primary patency of 86% and a procedural success rate of 93%.
The main argument for the use of a covered stent rather than an uncovered stent is that the layer material will provide a direct barrier to tissue ingrowth from neointima hyperplasia.25 However, restenosis can develop at the edges not coverd by graft material where intima injury may have occurred.26,27
Interim results from the Covered versus Balloon Expandable Stent Trial conducted in Australia have provided excellent results for challenging aortoiliac occlusive disease.28 Patients with classification of TASC B, C, or D in the iliac level were randomized to the Advanta V12 group versus a bare metal stent group. They were followed with duplex ultrasound at various intervals and freedom from stent occlusion was assessed. The covered stent group had significantly lower restenosis rate and greater freedom from stent occlusion than the bare metal stent group. The covered stent also showed superiority when treating TASC C and D lesions.
Indications for Stent Placement
Indications approved by the FDA for iliac stent placement include stenotic or occlusive atherosclerotic lesions or failed or inadequate balloon angioplasty in the iliac artery (Box 22-1). The latter condition can be assessed by angiography, intravascular ultrasound (IVUS), or hemodynamic means. Angiographic or IVUS detection of residual stenosis of 30% or greater, unstable intimal flaps, and dissection along the subintimal or medial layers are all considered inadequate angioplasty results that warrant intraluminal stent placement. Furthermore, a transstenotic pressure gradient of 10 mm Hg or greater following angioplasty is considered an indication for iliac stent placement. Provocative testing using pharmacologic agents that stimulate vasodilatation may be necessary to identify a hemodynamically significant lesion. Up to 75% of patients without a translesion pressure gradient will have a significant gradient following injection of a vasodilator. Either 100 to 200 µg/mL of nitroglycerin or 30 to 60 mg of papaverine can be injected directly into the vessel in question. Immediate arterial dilatation is induced, thus mimicking exercise. A pressure drop of 10 mm Hg or more is considered indicative of a hemodynamically significant stenosis.
Box 22-1
Indications to Intravascular Stent Placement
In addition to the FDA-approved indications for iliac artery stenting, certain other arterial conditions may be considered for stent placement. In the case of early restenosis following balloon angioplasty, stent placement is appropriate rather than repeated angioplasty alone. The 2-year patency rate of the iliac artery following angioplasty is between 65% and 81% and is affected by numerous factors, such as the degree of stenosis, the length of narrowing, and the distal vessel patency.3,29,30 Restenosis of the iliac artery frequently occurs at the original angioplasty site,7,9,11,31 which can be caused by neointimal hyperplasia or rapid atherosclerotic progression. Iliac artery stenting in such a condition can delay the restenotic process.
Primary stent placement, as opposed to stenting following inadequate angioplasty, has been used more frequently in the treatment of difficult iliac lesions. Nearly or totally occluded iliac arteries may be appropriate for primary stenting because angioplasty alone in these situations generally yields poor long-term patency rates.14,32–36 Stenotic iliac vessels with ulcerative plaques can cause distal embolization when treated with balloon angioplasty.6,37 It is appropriate to perform primary stent placement in such a condition to prevent plaque dislodgment. Moreover, primary stenting can be used as an adjunct procedure to improve iliac inflow when combined with a planned infrainguinal bypass operation.38–40 A randomized controlled trial comparing primary stent placement versus primary angioplasty followed by selective stent placement found similar cumulative patency at 2 years (71% vs. 70%, respectively; p = 0.6).41 The authors concluded that because angioplasty followed by selective stent placement is less expensive than primary stent placement, the former seems to be the treatment of choice for intermittent claudication owing to iliac artery occlusive disease.
Contraindications to Stent Placement
Box 22-2 summarizes the contraindications for intravascular stent placement. Arterial perforation as a result of balloon angioplasty, as evidenced by contrast extravasation, is considered a contraindication for intraarterial stent placement. Stent placement in such a condition can lead to severe hemorrhagic complications or pseudoaneurysm formation. In these challenging situations, placement of a covered stent may be more appropriate.42,43 Stent placement should also be avoided in aneurysmal arteries, because persistent blood flow around the stent can lead to further aneurysm expansion unless a covered stent is used. Because balloon-expandable stents, such as the Palmaz stent, are relatively rigid, they are less than ideal in tortuous vessels. Self-expanding stents, such as the Wallstent, have greater flexibility and are more appropriate in vessels with marked tortuosity. Severely calcified arteries often are not amenable to balloon angioplasty; similarly, stent placement should not be expected to restore the normal vessel lumen, because stent deployment requires the same basic techniques as balloon angioplasty. Long-standing arterial occlusion is considered a relative contraindication to stent placement. In this setting, the risk of plaque embolization resulting from stent expansion may outweigh the potential benefit of arterial recanalization. Finally, stent deployment should be avoided in arteries that might serve as either the proximal or distal site of a bypass grafting procedure. In a stented artery, application of vascular clamps might not only cause significant damage to the arterial wall, but also crush the stent so that reexpansion is impossible. If vascular control is needed in a stented artery, intraluminal occlusion balloon catheter should be used rather than vascular clamps.
Box 22-2
Contraindications to Intravascular Stent Placement
• Arterial perforation with contrast extravasation in the target vessel (covered stent may treat this complication)
• Marked vessel tortuosity (not a contraindication for Wallstent placement)
• Target lesions cross areas of flexion, such as the inguinal ligament, knee, or shoulder
• Coexistent aneurysmal disease requiring surgical intervention
• Successful balloon angioplasty
• Presence of hypercoagulable disorder
• Long-standing arterial occlusion
• Target vessels that may serve as the proximal or distal site of a bypass grafting procedure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree