Aneurysms and Aortic Dissection
Management of arterial aneurysms and aortic dissection requires an understanding of natural history, diagnosis, and treatment options. In recent years, these options have changed dramatically with the development of endovascular techniques. Despite these changes, the best results continue to follow carefully planned elective treatment before complications of rupture, thrombosis, or embolism occur. The contrast in mortality between elective (2-5%) and ruptured abdominal aortic aneurysm (AAA) repair (50-70%) remains one of the most striking examples of the importance of early recognition and proper treatment of these diseases.
Aortic dissections are a commonly encountered degenerative disease of the aorta, which are distinctly different from aortic aneurysms. This chapter focuses on the natural history, diagnosis, and management of aneurysms and aortic dissections. The hemodynamics of aneurysms are discussed in Chapter 1. Chapter 4 outlines the initial physical evaluation, and Chapter 6 outlines several useful diagnostic tests that may be used to diagnose and follow these conditions.
ABDOMINAL AORTIC ANEURYSM
I. Epidemiology.
During the past 30 to 40 years, the incidence of AAAs has increased significantly. This is attributed to increased detection with the use of ultrasound and computed tomography (CT) and an aging population. The rising incidence has been tenfold for small AAAs (<5 cm), while the incidence for larger aneurysms has increased by a factor of 2. Small aneurysms account for 50% of all recognized AAAs, an important consideration given that much of the uncertainty surrounding appropriate management concerns aneurysms <5.5 cm.
II. Natural history and the evolution of evidence-based approach.
Aortic aneurysms are a disease of the elderly diagnosed in the sixth and seventh decades of life. As many as 20% of patients with an AAA have a family history of aortic aneurysm. The expansion rate of AAAs is 2 to 3 mm per year and increases as the aneurysm enlarges. Twenty percent of aneurysms expand at a rate of more than 4 mm per year, while 80% grow at a slower pace. Importantly, active cigarette smoking has been shown to be associated with an increased expansion rate and has been identified as an independent risk factor for aneurysm rupture.
The natural history of AAA is expansion and rupture, an outcome that is related directly to aneurysm diameter. Less commonly, enlarging aneurysms may erode into the vena cava resulting in an aortocaval fistula, or into the intestine presenting as gastrointestinal bleeding (i.e., aortoenteric fistula). These concepts are controversial, not because of the risk associated with open surgery, but because experts have debated the
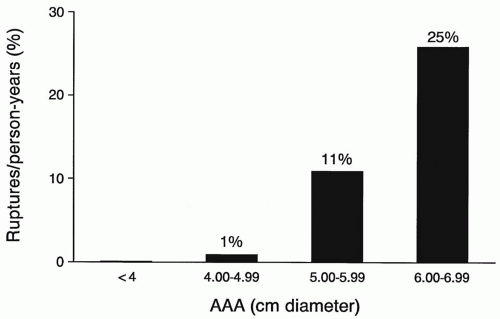
size at which repair should occur. Initially, aneurysms <6 cm were considered appropriate for elective repair. Autopsy studies in the 1970s and 1980s suggested that even small aneurysms (4 to 5 cm) could rupture, which resulted in a more aggressive approach to repair. Population-based studies in the 1990s showed that rupture risk did not increase until aneurysm diameter reached 5 cm (Figs. 15.1 and 15.2). Rupture risk for small (<5 cm) AAAs was shown to be approximately 1% per year, 5% to 10% per year for medium-sized (5 to 7 cm) AAAs, and at least 10% to 25% per year for large (7 cm) AAAs. The more recent U.K. Small Aneurysm and Aneurysm Detection and Management (ADAM) trials were prospective randomized studies looking for survival benefit in early open repair of aneurysms between 4 and 5.4 cm. Findings from these trials confirmed the population-based studies of the 1990s, while showing no benefit to early open repair of aneurysms between 4 and 5.4 cm. Several caveats from these studies are worth noting. First, safe observation of aneurysms between 4 and 5.4 cm includes ultrasound every 3 to 6 months, which many feel is unrealistic for some patients. Second, in both studies two-thirds of patients in the “observation” group crossed over to eventual open repair once their aneurysms reached 5.5 cm during the study period. Finally, in this era of endovascular aneurysm repair (EVAR) it is important to remember that both the U.K. Small Aneurysm and the ADAM trial compared observation to early open aneurysm repair and not to EVAR. What makes this relevant is that the mortality associated with open repair in these two trials was 5.8% and 2.7% respectively, which is higher than recently reported for EVAR.
size at which repair should occur. Initially, aneurysms <6 cm were considered appropriate for elective repair. Autopsy studies in the 1970s and 1980s suggested that even small aneurysms (4 to 5 cm) could rupture, which resulted in a more aggressive approach to repair. Population-based studies in the 1990s showed that rupture risk did not increase until aneurysm diameter reached 5 cm (Figs. 15.1 and 15.2). Rupture risk for small (<5 cm) AAAs was shown to be approximately 1% per year, 5% to 10% per year for medium-sized (5 to 7 cm) AAAs, and at least 10% to 25% per year for large (7 cm) AAAs. The more recent U.K. Small Aneurysm and Aneurysm Detection and Management (ADAM) trials were prospective randomized studies looking for survival benefit in early open repair of aneurysms between 4 and 5.4 cm. Findings from these trials confirmed the population-based studies of the 1990s, while showing no benefit to early open repair of aneurysms between 4 and 5.4 cm. Several caveats from these studies are worth noting. First, safe observation of aneurysms between 4 and 5.4 cm includes ultrasound every 3 to 6 months, which many feel is unrealistic for some patients. Second, in both studies two-thirds of patients in the “observation” group crossed over to eventual open repair once their aneurysms reached 5.5 cm during the study period. Finally, in this era of endovascular aneurysm repair (EVAR) it is important to remember that both the U.K. Small Aneurysm and the ADAM trial compared observation to early open aneurysm repair and not to EVAR. What makes this relevant is that the mortality associated with open repair in these two trials was 5.8% and 2.7% respectively, which is higher than recently reported for EVAR.
In 1997 the U.S. FDA approved the first device for EVAR. More than a decade later, the expanded use of this procedure seems to have had a positive impact on the mortality associated with AAA repair. Information from the Medicare database recently revealed that in the first 3 years of this decade the number of AAA repairs in the United States had not changed (approximately 60,000 per year). However the percentage of AAAs treated with EVAR did increase and, with an operative mortality of less than 2%, this less invasive approach appears to have reduced the overall mortality associated with AAA repair; something that had not been possible in this country for decades. How this shift in the treatment of AAAs impacts recommendations for repair in relation to AAA size remains to be seen and is the topic of significant clinical and epidemiologic research.
From these decades of clinical study, one can be assured that nearly all AAAs that rupture have enlarged to over 5 cm in diameter. Unfortunately, the prevalence of rupture remains unchanged, and even today 70% of patients with ruptured AAA are unaware of the diagnosis until the day of rupture. This statistic has been used as an important rallying cry for those advocating for the wider use of ultrasound screening to detect AAAs in targeted populations.
III. Indications for operation.
A. A practical approach using aneurysm diameter.
Currently in our practice, patients with asymptomatic AAAs <5 cm are followed with ultrasound. For these patients, CT scans are typically not obtained and aneurysm repair not recommended unless part of a clinical trial or if the most recent measurement confirms rapid expansion (i.e., more than 6 mm in 6 months or more than 1 cm in one year). Aneurysms <5 cm may occasionally be repaired as part of treatment for symptomatic aortoiliac occlusive disease with open (e.g., aortobifemoral bypass) or endovascular techniques.
Good-risk patients with aneurysms between 5 and 5.4 cm receive a contrast-enhanced aortic CT to define the size, shape, and extent of the aneurysm. Such patients are provided a summation of our clinical understanding of aneurysm observation versus repair, open and EVAR. Patients are made aware that although it is safe to observe aneurysms <5.5 cm, close surveillance will be required and it is likely that within 1-3 years the aneurysm will expand and repair will be required. Repair of asymptomatic aneurysms between 5 and 5.4 cm is not rushed and is strongly influenced by individual patient factors, such as medical comorbidity (e.g., risk of anesthesia), ability to commit to close surveillance, candidacy for EVAR, and level of patient anxiety regarding the aneurysm. If these individual factors weigh in favor of repair, good-risk patients with AAAs between 5 and 5.4 cm are offered an operation either open or EVAR. Finally all good risk patients who present with an aneurysm of 5.5 cm or larger receive a contrast-enhanced aortic CT and are offered repair, open or EVAR, in an expeditious manner.
For high-risk patients or those with more limited life expectancy and aneurysms greater than 5 cm we defer to less invasive EVAR if aneurysm morphology permits. If such high-risk patients are not endovascular candidates, we most often delay any open operation until the aneurysm expands to 6 cm or becomes symptomatic.
B.
A less common but more urgent indication for AAA repair is evidence of peripheral emboli in the lower extremities of patients with aneurysms.
C. Urgent aneurysm repair is also indicated for patients with a known aneurysm that has become tender or is associated with abdominal or back pain.
These patients should be hospitalized and considered to have a symptomatic aneurysm, even though their vital signs may be normal and their abdominal symptoms nonspecific.
D. Patients with ruptured aneurysms and shock should be taken directly to the operating room for resuscitation and operation.
An increasing number of series now report the treatment of ruptured AAAs by endovascular means (i.e., EVAR), an option that has become more viable with increased experience, improved devices, and more complete “on-the-shelf” endovascular inventories.
IV. Preoperative evaluation.
The preoperative evaluation for an elective AAA should define the size and extent of the aneurysm, associated medical risks, and associated vascular disease.
A. Size and extent of AAA.
The reliability of the abdominal exam to detect and measure an AAA is poor. Information from the ADAM trial showed that the accuracy of the physical exam to detect an aneurysm in an individual with an abdominal girth of 38 inches or more was around 50%. The simplest and least expensive test to diagnose and measure an AAA is ultrasound. Measurement of the anterior-posterior diameter is more accurate than the transverse diameter; reliably measuring to within 2-3 mm. Ultrasound is also the favored method to follow changes in aneurysm diameter over time (i.e., aneurysm surveillance).
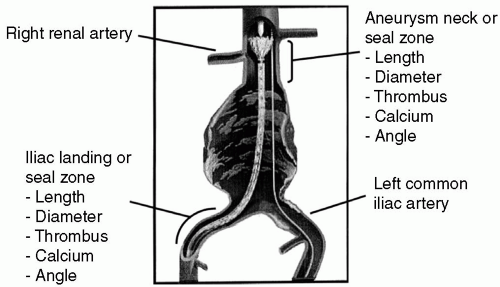
Although some prefer routine CT scan for patients with suspected or known small AAAs, this imaging modality costs more than ultrasound and carries morbidity associated with contrast administration. In our practices, contrast-enhanced aortic CT is reserved for aneurysms that are >5 cm or in which repair is actively being planned. In addition to assessing the size of an AAA, a common reason to obtain a CT is anatomic evaluation for EVAR (Figure 15.3). In this regard, CT assesses the following characteristics of the proximal aortic and distal iliac artery seal or landing zones where the endograft is fixed, thereby excluding the aneurysm from flow and pressure:
The diameter and length in relation to branch arteries (e.g., renal and internal iliac arteries)
The degree of circumferential calcification and/ or thrombus
The degree of tortuosity or angle
The length of aneurysm in relation to the aortic bifurcation and branch arteries
In addition, an aortic CT is indicated when a suprarenal or thoracoabdominal aneurysm is suspected. One must remember that CT scan may occasionally overestimate the diameter of an aneurysm because the measurements are made perpendicular to the body axis, which introduces some error if the aorta is tortuous. This error can now be corrected for by use of modern CT software, which allows for calculations referred to as center line measurements of aneurysm length and diameter.
B.
Aortography is not reliable for determining aneurysm diameter, as luminal thrombus obscures the outer limit of the aneurysm wall. Aortography prior to aneurysm repair, open or endovascular, should be used selectively for the following criteria:
Decreased peripheral pulses or symptoms of lower-extremity claudication
Poorly controlled hypertension or renal insufficiency indicating renal artery occlusive disease
Suprarenal or thoracoabdominal aortic aneurysms requiring delineation of visceral and intercostal arteries
Symptoms of intestinal ischemia suggesting visceral artery occlusive disease
Suspected horseshoe kidney with multiple renal arteries on ultrasound or CT
Although once commonplace, aortography prior to EVAR is now rarely indicated because of the high quality of dynamic, contrast-enhanced aortic CT. One or more of these criteria for preoperative aortography is present in fewer than 10% of patients with AAA, making this invasive preoperative test uncommon.
C. Medical risks.
Most medical comorbidities can be detected by the performance of a thorough history and physical exam, both of which are outlined in more detail in Chapters 4 and 7. As many as 50% of patients with AAAs have some degree of coronary artery disease. Evidence-based guidelines offered by the American Heart Association (AHA) and the American College of Cardiology (ACC) in regard to evaluation prior to AAA repair are outlined in Chapter 8. These guidelines include recommendations for preoperative cardiac testing and selective preoperative cardiac revascularization. In the context of these guidelines, traditional open aortic aneurysm repair is categorized as a high-risk procedure (see Table 8.5) because of the need for an abdominal or retroperitoneal incision and cross-clamping of the aorta. Endovascular aneurysm repair should be considered an intermediate risk procedure (see Table 8.5) as it may be done under regional anesthesia, does not require aortic cross-clamp, and can even be performed percutaneously in many cases. In our experience, adherence to the AHA/ACC Clinical Guidelines contributes to a low elective operative mortality for open (3-4%) and endovascular (<2%) AAA repair.
A more aggressive approach to AAA repair in older or high-risk patients has been advocated by some. EVAR now clearly offers an option that is safer for many of these patients although they must still be carefully selected and treated by an experienced anesthesia and endovascular team. Under these conditions, EVAR in high-risk patients can now be accomplished with an operative mortality of <5%. If not repaired, many of these high-risk patients with larger AAAs will succumb to aneurysm rupture.
D. Associated vascular disease. Approximately 10% of patients with an AAA will have associated carotid occlusive disease in the form of an asymptomatic carotid bruit or symptoms such as TIA or stroke.
In our practice,
these patients undergo carotid duplex to determine the significance of the carotid disease (Chapter 6). Patients with symptomatic carotid stenoses undergo expeditious treatment of the carotid prior to AAA repair even if the aneurysm is large. In these cases, the timing of the AAA repair depends on the size of the aneurysm and, unless it is quite large (>7 cm) or symptomatic, the aneurysm repair is performed a week or two after treatment of the symptomatic carotid. Patients with asymptomatic carotid stenoses may undergo elective AAA repair prior to carotid endarterectomy. Exceptions to this are patients with asymptomatic unilateral preocclusive stenosis or bilateral high-grade stenoses, in which case it is reasonable to treat the carotid prior to AAA repair. However, patients with symptomatic or large aneurysms (>7 cm) should undergo AAA repair without delay for carotid surgery. Overall, the risk of perioperative stroke from carotid occlusive disease during AAA repair is low, and clinical evidence supporting preoperative carotid treatment is lacking.
these patients undergo carotid duplex to determine the significance of the carotid disease (Chapter 6). Patients with symptomatic carotid stenoses undergo expeditious treatment of the carotid prior to AAA repair even if the aneurysm is large. In these cases, the timing of the AAA repair depends on the size of the aneurysm and, unless it is quite large (>7 cm) or symptomatic, the aneurysm repair is performed a week or two after treatment of the symptomatic carotid. Patients with asymptomatic carotid stenoses may undergo elective AAA repair prior to carotid endarterectomy. Exceptions to this are patients with asymptomatic unilateral preocclusive stenosis or bilateral high-grade stenoses, in which case it is reasonable to treat the carotid prior to AAA repair. However, patients with symptomatic or large aneurysms (>7 cm) should undergo AAA repair without delay for carotid surgery. Overall, the risk of perioperative stroke from carotid occlusive disease during AAA repair is low, and clinical evidence supporting preoperative carotid treatment is lacking.
V. Preoperative preparation for elective AAA repair is now occupied mostly by study of aortic CT reconstructions in consideration of EVAR.
Suitability for EVAR, type of endograft, and specific endovascular approach all require significant consideration and planning. To this end it is our practice to have more than one set of eyes evaluate the aortic CT prior to final decisions regarding EVAR. This is productive when in the form of discussion within our vascular and endovascular surgery group, but may also be accomplished with experienced radiologists or even trusted clinical specialists employed by industry. Careful evaluation of the aortic CT in these forums helps the endovascular specialist anticipate and plan for “trouble spots” during EVAR before starting the case. This type of disciplined preoperative preparation before EVAR maximizes the likelihood of a successful treatment and minimizes the chances of a misguided endovascular attempt.
VI. Management of a ruptured aneurysm.
Although EVAR has been utilized to treat ruptured AAAs, in some specialized institutions the most common form (>90%) of management remains emergent open repair. The key to successful management of a ruptured AAA with open or endovascular techniques is expeditious movement of the patient to the operating room. Delay in the emergency or radiology departments often results in deterioration and death from hemorrhage.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree