(1)
Department of Neuroscience, University of Turin Ospedale Molinette, Turin, Italy
Abstract
The precise incidence of cerebral aneurysms is unknown. From autopsy studies, the incidence is estimated at 5 % (Stehbens 1972, 1990).
11.1 Incidence
11.2 Type and Location
Cerebral aneurysms are commonly of a saccular type (berry aneurysm) located at the bifurcation of the arteries of the circle of Willis. The most frequent sites are the internal carotid artery (ICA; 30–35 %). Among them, those in the area of the junction of the posterior communicating artery (PcomA) account for more than the half. Also occurring very frequently are aneurysms involving the sector of the anterior cerebral artery (ACA; 33–34 %) and those of the middle cerebral artery (20 %).
Aneurysms in the posterior circulation are less common (10 %). Of these, half are basilar artery aneurysms (Locksley 1966a, b; Weir 1987; Nakstad et al. 1988).
Multiple cerebral aneurysms frequently occur together, with an incidence that varies from 20 to 40 % (Locksley 1966a, b; Nakstad et al. 1988; Osborn 1999; Rinne et al. 1994). Multiple aneurysms occur more frequently among women than men. It is thinkable that in those patients in whom there is a tendency to develop multiple aneurysms, these do not appear always simultaneously but in different period of life. This could also explain the appearance of the in the literature called “de novo” aneurysms sometimes even years after the diagnosis of a first aneurysm (Wermer et al. 2005). Furthermore, the presence of multiple aneurysms suggest that at least in these cases we are dealing not with a focal but with a diffuse progressing vascular disease of the cerebral vessels which needs to be monitored (Bruneau et al. 2011) (Fig.11.14c–f).
11.3 Macroscopic Appearance
Saccular aneurysms vary in size. In in vivo studies, the majority have a diameter of 5–7 mm, though smaller and larger aneurysms also occur; those larger than 25 mm are termed giant aneurysms. Saccular aneurysms can be round or more elongated. The surface may be regular or irregular with estroflexion (blebs), which in the case of a subarachnoid hemorrhage (SAH) can indicate the point of rupture. Pathological studies show that the neck and sac have no internal elastic lamina and no tunica media.
11.4 Pathogenesis
Intracranial cerebral vessels and intracranial aneurysms have some unique features. The arteries are composed of four layers, represented by the adventitia, media, internal elastic lamina, and intima. There is no external elastic lamina. The arteries have thin walls and are located in the subarachnoid space, where they have relatively little external support. Typical aneurysms of the intracranial cerebral vessels are saccular and thus different from aneurysms affecting the aorta or other extracranial arteries, which are commonly fusiform (Stehbens 1990; Powell 1991). In the early 1930s, some authors (Forbus 1930) showed in histological studies a congenital gap defect of the media in the wall of the cerebral artery, which was interpreted as a locus minoris resistentiae, where aneurysms could develop. Later, however, it was demonstrated that these gaps are very frequent in normal individuals without aneurysms. Today, it is commonly accepted that aneurysms are acquired lesions linked to a degenerative process involving the connective tissue of the media and internal elastic lamina, in which atherosclerosis probably plays an important role (Glynn 1940; Carmichel 1950; Crompton 1966a, b; Stehbens 1959, 1972, 1989, 1990). Others (Pope et al. 1984; Pope 1989; Ostergaard and Oxlund 1987; Chyatte et al. 1990) have demonstrated a reduction in reticular fibers owing to a decrease in type III collagen in the tunica media of cerebral arteries of patients with aneurysms; this is perhaps due to an undetected metabolic disorder of connective tissue proteins. In a few of these patients, genetic disorders could be demonstrated (Chyatte and Mjerzejewski 1991).
These degenerative changes lead to mural fragility, upon which hemodynamic factors, such as hypertension, variations in the circle of Willis, and arteriovenous malformation (AVM), could act further toward the formation of aneurysms. For aneurysms linked to AVM, see Chap. 12.
There are other more uncommon pathological conditions in which cerebral aneurysm can occur. Among them are a few systemic diseases of the connective tissue, such as fibromuscular dysplasia, Ehlers–Danlos syndrome, Marfan syndrome, neurofibromatosis, polycystic kidney, and aortic coarctation. In some of these patients, hypertension is also present as an adjuvant factor (Schwartz and Baronofsky 1960; Handa et al. 1970a; Stehbens 1989; Osborn 1999; Benyounes et al. 2011).
Aneurysms can occur in infectious diseases, arteritis, and cases of cardiac myxoma. Other factors reported to promote aneurysm formation are smoking, consumption of alcohol, and oral contraceptives (Hillbom and Kaste 1982; Lindergard et al. 1987; Juvela et al. 1993). The incidence of aneurysms is reported to be high in some families (Edelsohn et al. 1972; Hashimoto 1977; Crompton 1979; Norrgard et al. 1987).
Finally intracranial aneurysms can occur following a traumatic lesion of the arterial wall leading to dissection and to formation of a pseudoaneurysm (Birley and Trotter 1928; Benoit and Wortzman 1973; Jacobson et al. 1984; Bozzetto-Ambrosi et al. 2006; De Andrade et al. 2008; Nakstad et al. 2008). In the past, angiography was the method of choice to examine patients with craniocerebral trauma. Aneurysms could be easily detected. Today the diagnosis is performed with CT. With this method, in spite of the improved quality, small aneurysms can escape to the routine CT diagnosis. As reported also by others (Jussen et al. 2012) in patients with severe trauma especially in whom CT has shown an intracranial hemorrhage, a study of the vessels with CT angiography or conventional angiography is mandatory to exclude a vascular lesion especially an aneurysm of the cerebral or meningeal arteries. As far as it concerns aneurysms due to spontaneous dissection, see Chap. 16.
11.5 Clinical Presentation
Aneurysms are typical in patients of adult age, and there is predominance among women. Aneurysms in pediatric patients are rare; they are more frequent in boys. In 90 % of cases, the aneurysm presents with subarachnoid hemorrhage (SAH) (10 cases of SAH/year/100,000 people). On CT the bleeding in the subarachnoid space and in the ventricular system is commonly easily recognizable. Involvement of the adjacent parenchyma can occur especially in aneurysms of the middle and the anterior cerebral artery. Extension to the subdural space is sometimes recognizable in aneurysm near the skull basis. It is the merit especially of Dr. Symonds in the years 1923 and 1924 to identify some typical symptoms of the SAH and correlate these with a rupture of a cerebral aneurysm. Today this clinical condition with its typical sudden onset of headache, stiff neck, vomiting, and frequent disturbances of consciousness (drowsiness, confusion, and coma) is commonly easily recognized and correctly diagnosed. In some cases due to the very high increase of the intracranial pressure, death can ensue within minutes and hours. In other patients, the bleeding can be minimal (warning leak) and the mild clinical symptoms can be misinterpreted and the diagnosis of SAH can be eluded. Particular clinical aspects, which occur especially in severe SAH, are pulmonary complications and a myocardial dysfunction including arrhythmias, troponin elevation, myocardial infarction, and the syndrome called “neurogenic stunned myocardium” (Kono et al. 1994; Urbaniak et al. 2007; Frontera et al. 2008). All these cardiac complications can lead to cerebral infarct (Mayer et al. 1999; Gaita et al. 2002; Jain et al. 2004; Lee et al. 2006b). In other cases, the patient presents with neuropathy, particularly oculomotor palsy, in PcomA and basilar artery aneurysms. Trigeminal neuralgia can also occur in large aneurysms in this location. Visual symptoms, due to compression of the chiasma and/or optic nerve, and sometimes hydrocephalus, due to compression of the third ventricle, can be present in large aneurysms involving the anterior communicating artery (AcomA) and in carotid ophthalmic lesions. Symptoms as a result of brain compression can occur in large basilar artery and middle cerebral artery (MCA) aneurysms.
The typical cavernous sinus syndrome is present in intracavernous aneurysms.
In exceptional cases, aneurysms are present with ischemia. This occurs especially in nonruptured, partially thrombosed aneurysms (Antunes and Correll 1976; Stewart et al. 1980). Ischemia can be due to the distal embolization or involvement of perforators adjacent to the aneurysm.
In addition to aneurysms in patients presenting with related symptoms, an increasing number of asymptomatic aneurysms today are diagnosed with CT and MRI in patients who underwent the examination for other reasons. The clinical problems and therapeutic considerations with such aneurysms are discussed in Sect. 11.10.
11.6 Aneurysm Location
11.6.1 Extracranial ICA Aneurysms
Aneurysms in this location are uncommon (Fig. 16.3). Trauma is a possible etiology. Atherosclerosis can be another cause in older patients. In younger patients, aneurysms may be associated with FMD or collagenopathies, such as Ehlers–Danlos syndrome. In the latter syndromes, dissecting aneurysms are frequent.
11.6.2 Petrous Segment ICA Aneurysms
These are very rare. Atherosclerosis and dysplasia can be assumed to be the etiological mechanism (Fig. 11.1).
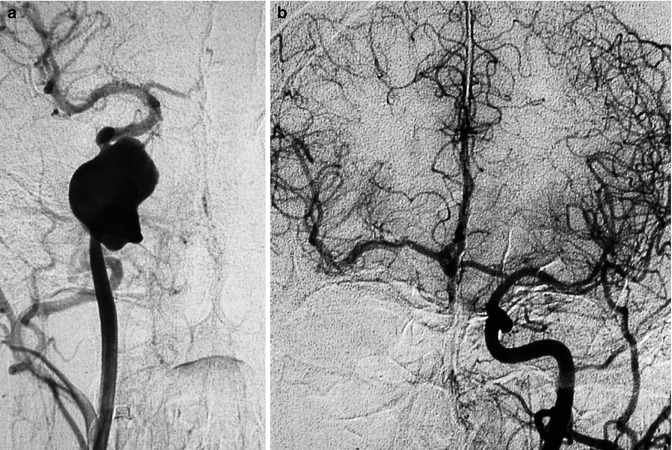

Fig. 11.1
Giant aneurysm of the petrous segment of the right internal carotid artery (ICA) presenting with palsy of cranial nerve VI. (a) Carotid angiogram showing the aneurysm, which was treated with occlusion of the ICA. (b) Left control angiogram, showing a good collateral circulation and exclusion of the aneurysm. The occlusion was preceded by ICA balloon test occlusion, during which an injection of the contralateral ICA was performed to examine the effectiveness of the circle of Willis and the appearance of the contralateral venous phase. In the tested territory, the venous phase should have a delay shorter than 2 s
11.6.3 ICA Paraclinoid Aneurysms
These are aneurysms arising adjacent to the anterior clinoid process. They can be divided into three groups: cavernous, ophthalmic, and superior hypophyseal.
Cavernous aneurysms are located in the cavernous sinus and are thus extradural. They can be asymptomatic or, when large, present with the typical cavernous sinus syndrome, first described by Jefferson, characterized by involvement of cranial nerves III, IV, VI, and, partially, V (Fig. 11.2). Commonly, there is no risk of SAH, and in general the clinical prognosis is good, and so in the treatment (endovascular occlusion of the ICA or selective occlusion of the aneurysm with stent plus coils and flow-diverter stents) size of the aneurysm, symptoms and age of the patient should be considered.
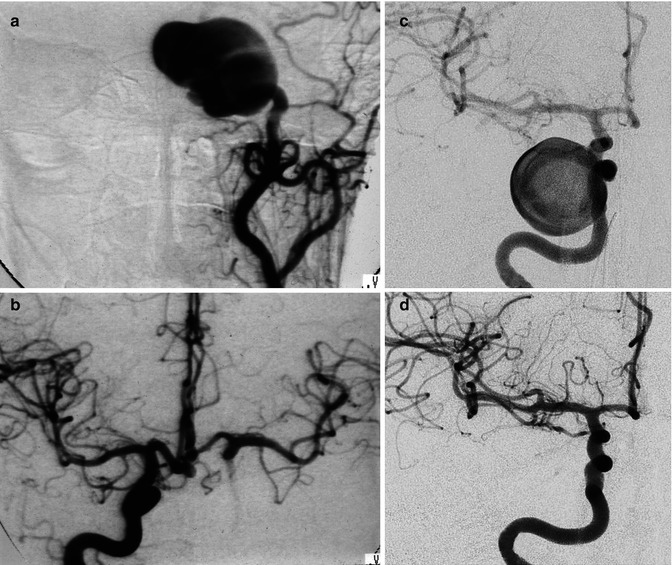

Fig. 11.2
(a, b) Giant aneurysm of the cavernous segment of the ICA presenting acutely with a typical cavernous sinus syndrome. Left carotid angiogram (a) showing the aneurysm. There is no injection of the intracranial branches. The right angiogram (b) showed a good collateral circulation through the circle of Willis. After a balloon test occlusion, as described in Fig. 11.1, the left ICA was occluded with a balloon. (c, d) Another patient with a giant aneurysm of the cavernous segment of the ICA presenting with a cavernous sinus syndrome treated with flow-diverter stent (silk). Angiogram before (c) and after (d) treatment
The aneurysms can rupture in the sphenoid sinus and nasal cavity, leading to a dramatic epistaxis. This is, however, an extremely rare possibility (Linskey et al. 1990). In some cases, intracavernous aneurysms, even small, can extend upward, reach the subarachnoid space, and rupture, causing SAH. This can occur since the dural ring surrounding the ICA, through which the ICA passes and becomes intradural, is medially not adherent to the artery; a small cavity (or cave) is thereby formed, through which the intracavernous aneurysm or those arising at the level of the medial ring can extend intradurally. This kind of aneurysm is called a cave aneurysm (Fig. 11.3).
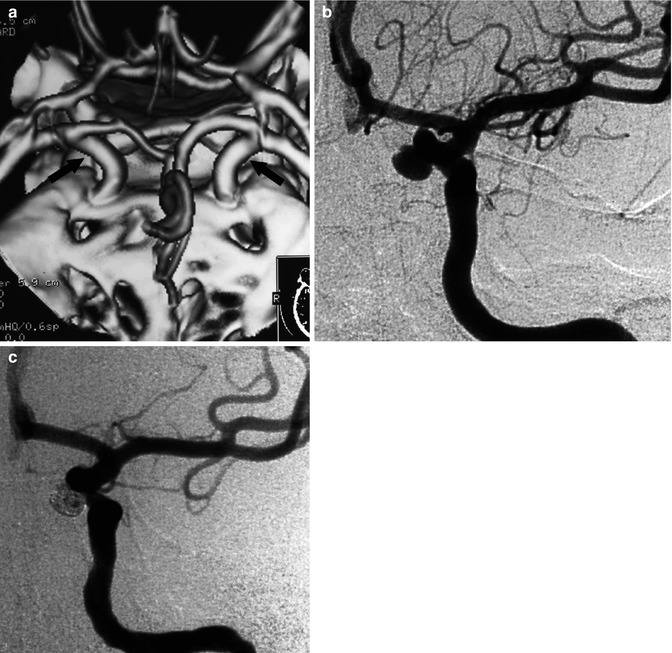

Fig. 11.3
Small cave left aneurysm presenting with hemorrhage. CT angiography (a) did not show the aneurysm. Both distal ICA (arrows). The left carotid angiogram (b) showed the small aneurysm, which probably had a small intradural estroflession control angiogram (c) posttreatment with coils
Carotid ophthalmic aneurysms arise in the ophthalmic segment of the ICA (see anatomy). They originate in association with the ophthalmic artery or its adjacent structures. They are directed upward, sometimes medially, and are frequently large (Figs. 11.4, 11.5, and 11.6).
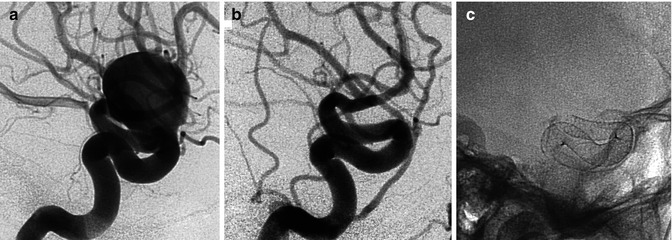
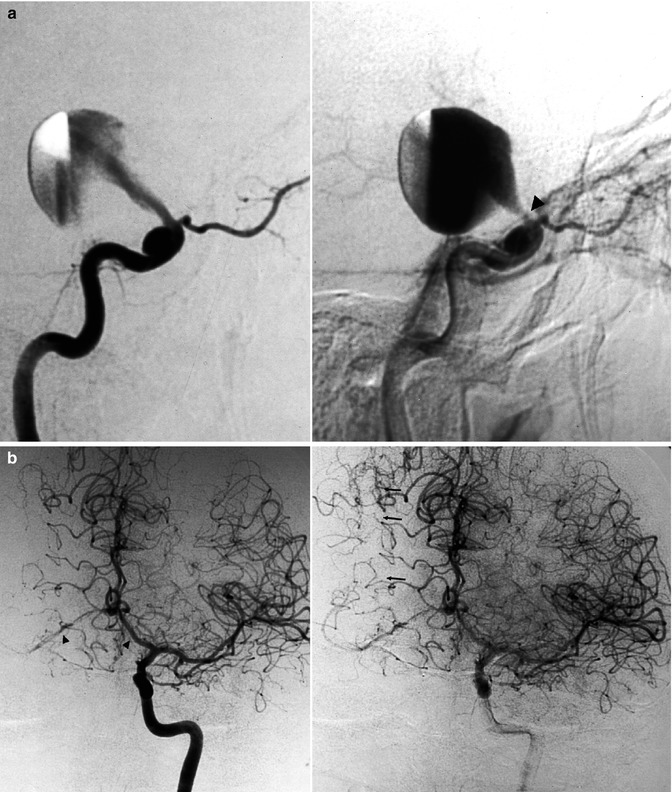
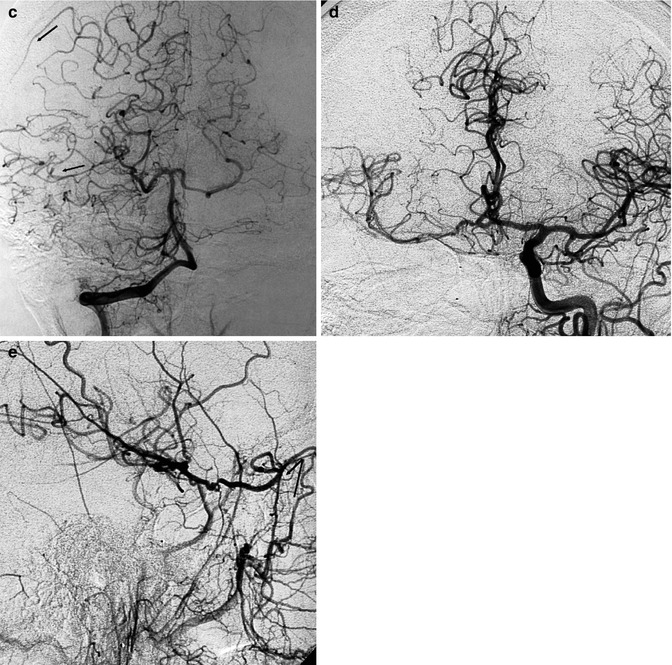
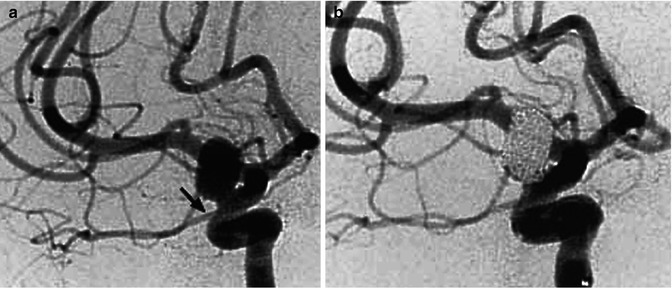

Fig. 11.4
Large carotid ophthalmic aneurysm presenting with progressively worsening visual acuity. A complete angiographic control to study the aneurysm and the circle of Willis was performed. The aneurysm was occluded with a flow-diverter stent. The patient improved slowly. Lateral carotid angiogram pretreatment (a), posttreatment (b). RX image showing the stent (c) (pipeline)


Fig. 11.5
Giant carotid ophthalmic aneurysm presenting acutely with severe disturbances of visual acuity. (a) Right lateral carotid angiogram, early and late phases, showing the progressive injection of the aneurysm through a relatively small neck, close to the origin of the ophthalmic artery (arrowhead). A second small intracavernous aneurysm is present. (b) Left carotid angiogram, upward displacement (arrowheads) of both A1 segments and partial filling of the right middle cerebral artery (MCA) through leptomeningeal anastomoses (arrows) with the right anterior cerebral artery (ACA). (c) Right vertebral angiogram, showing the leptomeningeal collateral circulation toward the right MCA from the posterior cerebral artery (PCA; arrows). (d) The ICA was occluded with a balloon positioned in the intracavernous segment. The patient recovered completely and a left control angiogram 2 months later showed the normal course of both the A1 and filling of the right MCA. (e) Right common carotid angiogram showing the retrograde injection of the distal ICA and MCA through the ophthalmic artery via a collateral circulation involving an anastomosis between the anterior deep temporal artery and the lacrimal branch of the ophthalmic artery (angled arrow). The aneurysm is completely excluded

Fig. 11.6
Carotid ophthalmic aneurysm presenting with hemorrhage. (a) Carotid angiogram, oblique view showing the aneurysm. Origin of the ophthalmic artery (arrow). (b) Carotid angiogram showing the occluded aneurysm with coils
Superior hypophyseal aneurysms are very rare. They arise in conjunction with the superior hypophyseal artery. They are directed downward and medially toward the chiasma and sella turcica, becoming intrasellar when large (Fig. 11.7).
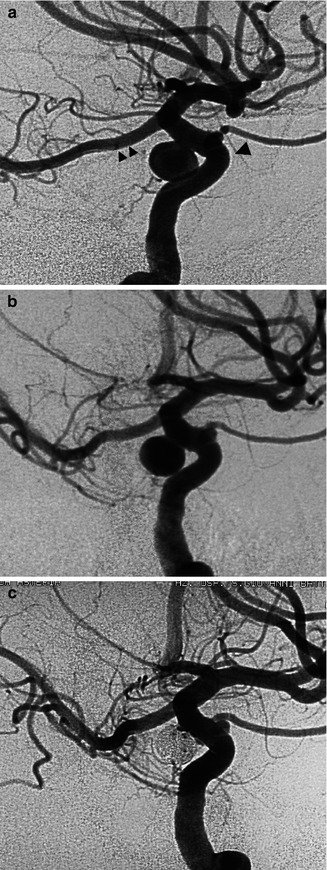

Fig. 11.7
Superior hypophyseal aneurysm presenting with hemorrhage. Two different oblique (a, b) views of the carotid angiogram to better identify the aneurysm and its neck. PCA (arrowheads), ophthalmic artery (arrowhead). Control angiogram (c) after occlusion of the aneurysm with coils
11.6.4 Aneurysms of the Communicating and Choroidal Segments
These consist of aneurysms of the PcomA and anterior choroidal artery (AchA). PcomA aneurysms arise at the origin of the PcomA; they are posteriorly directed and can compress cranial nerve III. They are frequently large, elongated, and with an irregular wall (Fig. 11.8a, b). More rarely aneurysms can arise selectively from the trunk of the PcomA (True PcomA aneurysms, Fig. 11.8c, d) (He et al. 2011). In some of these later cases, the etiopathogenesis is probably a dissection; involvement of the anterio-thalamoperforating branches is frequent (Nakao et al. 2004; Duncan and Terblanche 2005; Kocak et al. 2013).
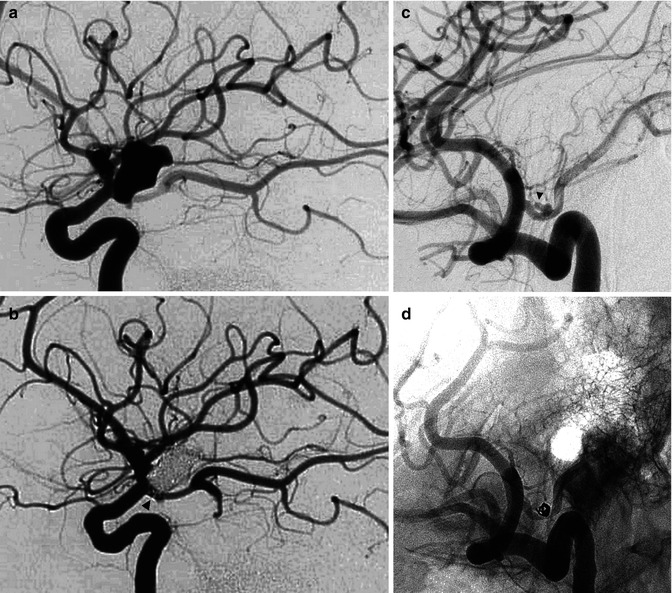

Fig. 11.8
(a, b) Large irregular aneurysm with a neck close to the origin of a fetal PCA in a patient with subarachnoid hemorrhage (SAH). (a) Lateral carotid angiogram showing the irregularly shaped aneurysm. The inferior part of the aneurysm is superimposed on the origin of the fetal PCA. (b) Lateral carotid angiogram. Occlusion of the aneurysm with coils. The origin of the PCA is now better recognizable (arrowhead). (c, d) Carotid angiogram, oblique view, showing (c) small irregular aneurysm located on PcomA (arrow head). (b) Control angiogram post-coiling
AchA aneurysms arise at the origin or just distal to the AchA (Fig. 11.9). They are directed posteriorly and laterally and are frequently smaller than PcomA aneurysms. A variable number of perforators arise from the choroidal segment of the ICA and can be found stretched around the neck of the aneurysm. In endovascular treatment, it is sometimes difficult to separate the neck of the aneurysm from the parent artery despite the use of several projections. Furthermore, the aneurysm can arise directly from the first segment of the AchA.
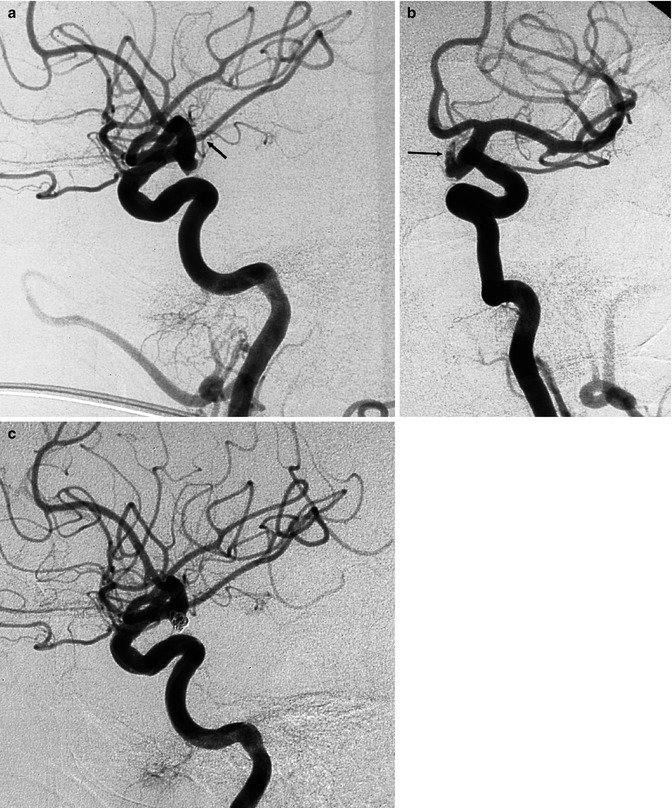

Fig. 11.9
Irregular ruptured aneurysm with neck close to the origin of the anterior choroidal artery (AchA). (a) Lateral carotid angiogram, showing the small aneurysm. AchA (arrow). (b) Oblique view better showing the irregular and elongated part (arrow) of the aneurysm. (c) Lateral carotid angiogram, posttreatment showing the occluded aneurysm with coils
11.6.5 Aneurysms of the Carotid Bifurcation
These are typical T-bifurcation aneurysms similar to basilar-tip aneurysms (Ingebrigtsen et al. 2004; Van Rooij et al. 2008). They are relatively rare and easily recognizable on the angiogram. They can sometimes be very large and have a sizeable neck. Perforating branches arising from the choroidal segment and from A1 and M1 may be stretched around the neck and posterior wall of the aneurysm (Figs. 11.10 and 5.4b).
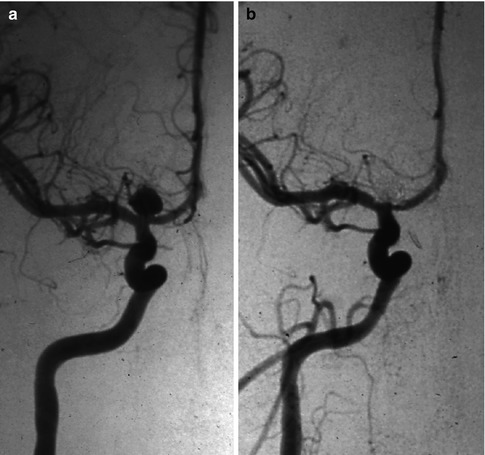

Fig. 11.10
Aneurysm of the distal carotid bifurcation, presenting with hemorrhage. (a) Carotid angiogram, AP view, showing the aneurysm. (b) Control angiogram showing the occluded aneurysm with coils
11.6.6 Anterior Cerebral Artery Aneurysms
These are very frequent aneurysms and arise mainly in the A1–A2 angle of the ACA. The neck of the aneurysm can be large and partially involve the adjacent A1–A2 segments or AcomA. As has been described in the anatomy, these aneurysms are frequently associated with anomalies of the anterior part of the circle of Willis, furthermore, many perforators arise from the A1 and AcomA. This should be taken into account in the surgical or endovascular treatment of these lesions (Figs. 4.4, 4.8, 4.9, 4.10, 4.11a, 4.12, 4.14, and 11.11). Infrequently, the aneurysm can be found along the A1 segment. In such cases, it can be assumed that the aneurysm takes its origin at the junction with a perforating branch. This has been confirmed in a large study describing the angiographic and surgical finding of these aneurysms (Suzuki et al. 1992).
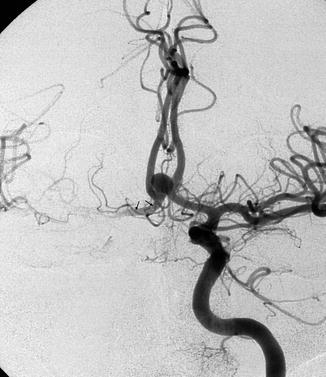

Fig. 11.11
Anterior communicating artery (AcomA) aneurysm. Carotid angiogram, AP view. The right artery of Heubner (arrows) is evident. On the left, it cannot be identified with certainty owing to the presence of other branches
Another typical location, though more uncommon, is at the pericallosal-calloso-marginal junction. Depending on the site of the junction, the aneurysms can be located anterior to the genu of the corpus callosum, distal to it, or, more rarely, proximal beneath the genu (Figs. 4.13 and 11.12). Dissecting, mycotic, “flow-dependent” aneurysms are very infrequent. They can occur in every segment of the ACA, usually in its distal segments (Figs. 11.13 and 16.10).
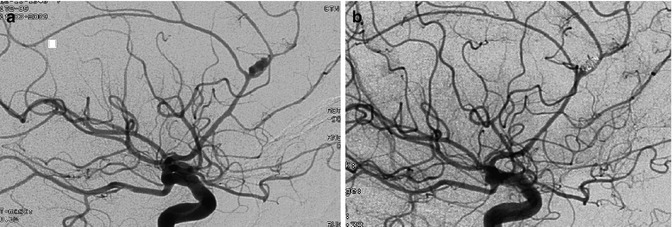
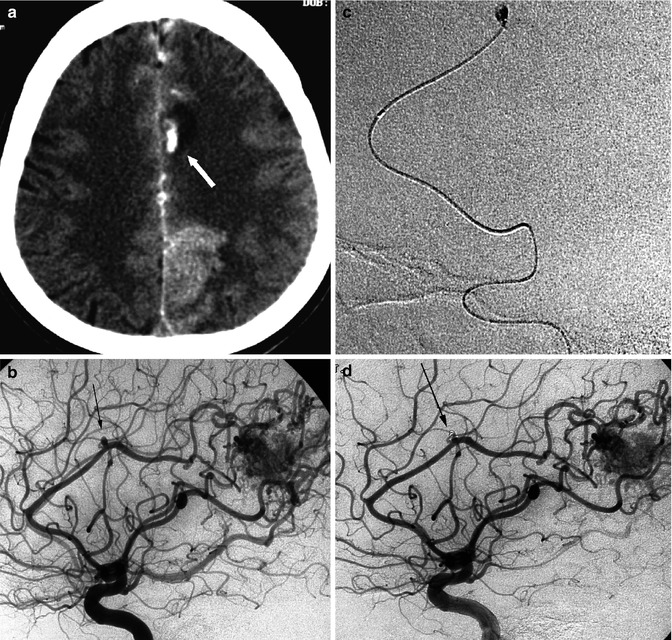

Fig. 11.12
(a) Lateral angiogram showing the typical site of a peripheral aneurysm of the ACA at the pericallosal-calloso-marginal junction. (b) Control angiogram posttreatment with coils

Fig. 11.13
Patient with known parietal AVM presenting with minimal inter-emispheric SAH and small haemorrhage involving the parenchyma not related to the AVM. (a) CT demonstrating the small haemorrhage with surrounding edema (arrow). (b) The angiography showed small pericallosal probably “flow-dependent” aneurysm (arrow). (c) Selective catheterization of the aneurysm occluded with coils. (d) Final control angiogram showing the occluded aneurysm (arrow)
11.6.7 MCA Aneurysms
Most of these aneurysms arise at the bi-trifurcation of the M1 (Fig. 11.14). It is not always easy to separate the bifurcation branches from the neck of the aneurysm. Furthermore, in cases of early division of the M1 (short M1), perforators can be very close to the aneurysm (Figs. 5.4c–d, 5.5a, e, and 5.12). Despite improvements in endovascular techniques, surgery remains the method of choice in many cases.
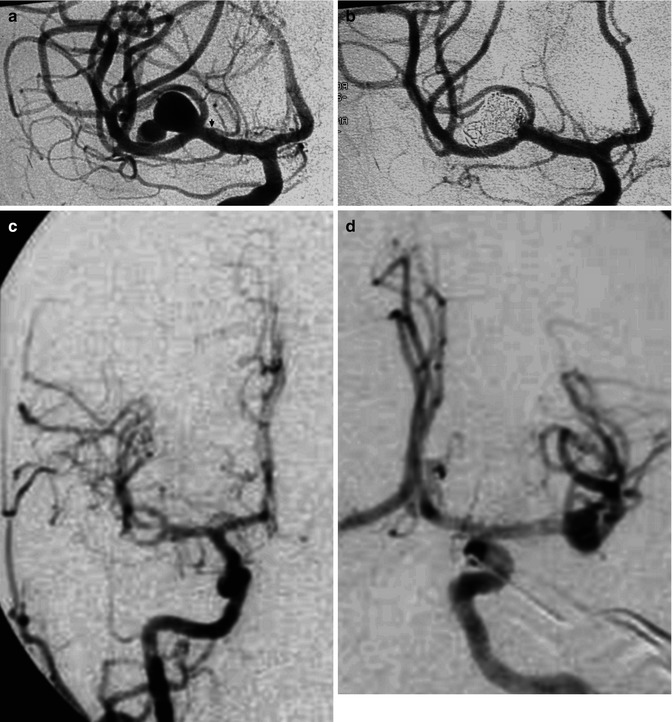
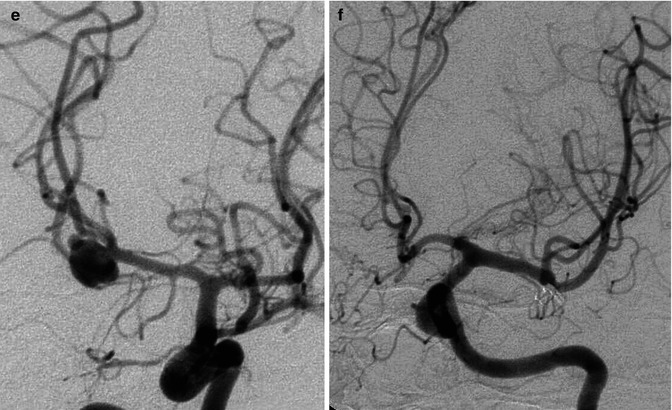


Fig. 11.14
Bilobated large saccular aneurysm at the MCA bifurcation, presenting with hemorrhage. Origin of the distal perforator close to the aneurysm (arrow). Pretreatment angiogram (a), posttreatment with coils (b). Another example of MCA aneurysm in a young patient (c–f) presenting with SAH due to rupture of a middle cerebral aneurysm visible on the left carotid angiogram (d) which was treated by surgery. Normal right carotid angiogram (c). About 8 years later the patient suffered a new SAH. There was no injection of the left aneurysm (f), but a new aneurysm was now recognizable on the right (e)
11.6.8 Aneurysms of the Posterior Circulation
Basilar aneurysms are the most frequent in the vertebrobasilar sector. They are typically located at the top of the artery. The perforators can shift and be stretched along the sac, but they are not directly involved since they arise from the P1; this can, however, occur when the neck enlarges and also involves the proximal part of the P1 (Figs. 11.15, 11.16, and 11.17). Other locations are the distal lateral basilar artery between the origin of the posterior cerebral artery (PCA) and the SCA (Fig. 11.18). Also typical are aneurysms of the trunk of the basilar artery, more frequently distal to or at the level of the anterior inferior cerebellar artery (AICA). Frequently, the latter aneurysms are relatively large, with a fusiform aspect, often due to dissection (Fig. 11.26).
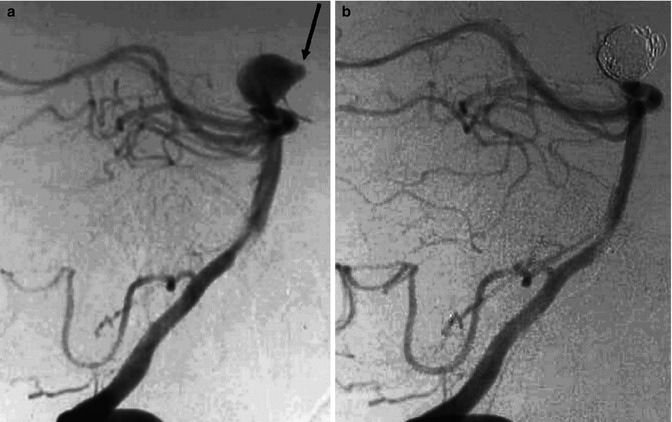
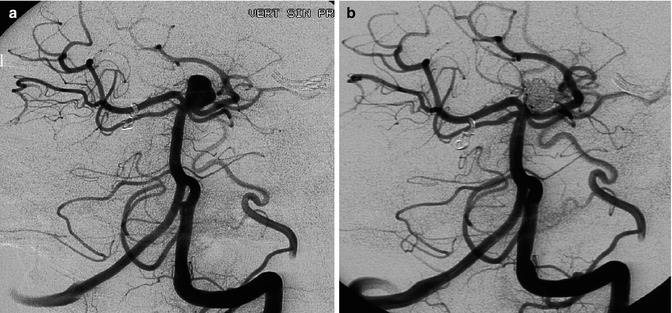
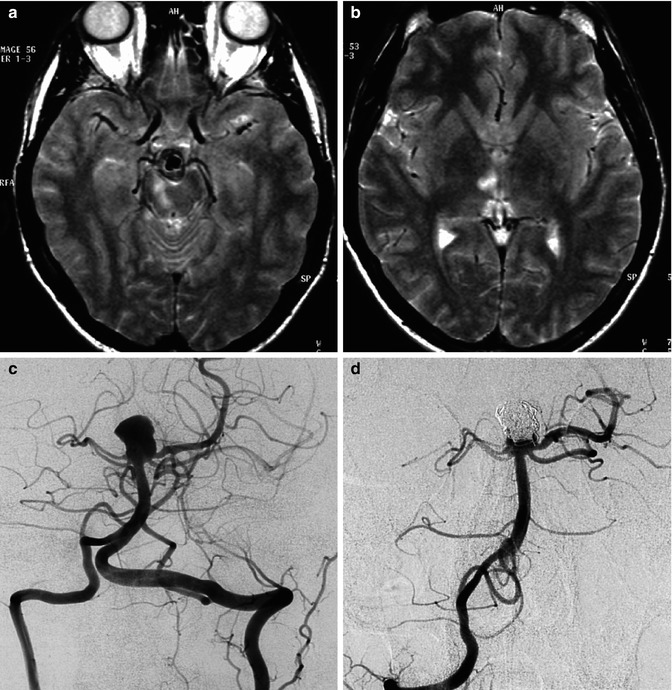
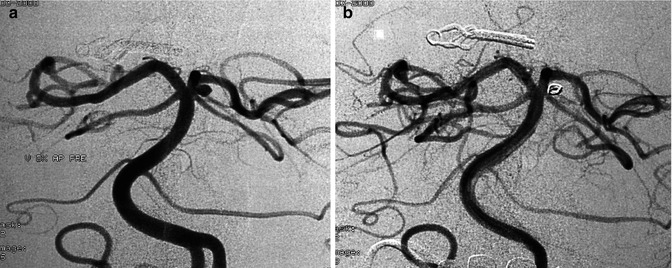

Fig. 11.15
Patient presenting with SAH. Vertebral angiogram (a) showing basilar-tip aneurysm with a bleb (arrow) probably indicating the site of rupture. Control angiogram (b) posttreatment with coils

Fig. 11.16
Nonruptured basilar-tip aneurysm with a neck involving partially also the left P1 segment. The patient had already been treated for a ruptured aneurysm of the AcomA. (a) Angiographic study pretreatment. (b) Control angiogram posttreatment. Occlusion with coils and stent

Fig. 11.17
A 40-year-old man with acute midbrain–thalamic syndrome. On MRI (a, b) small ischemic lesions were recognizable in the medial midbrain and medial thalamus on the right, corresponding to the vascular territory of perforators arising from the P1. A large basilar-tip aneurysm, probably responsible for the thromboembolic occlusion of the homolateral P1, was diagnosed (c). The patient recovered and was treated endovascularly a week later (d)

Fig. 11.18
A patient already operated on for an AcomA aneurysm. A second aneurysm was present at the junction of the left PCA and SCA. Vertebral angiogram (a). Control angiogram (b), post-coiling
Vertebral–Posterior Inferior Cerebellar Artery (PICA) Aneurysms. The vertebral–PICA junction is another typical site of aneurysms. These aneurysms are sometimes located not exactly at the junction, but close to it, in the proximal part of the PICA (Mukonoweshuro et al. 2003; Bradac and Bergui 2004; Mericle et al. 2006; Cellerini et al. 2008) (Figs. 11.19 and 11.20).
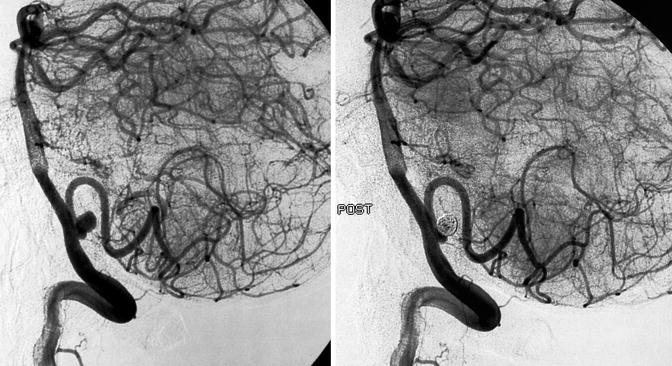
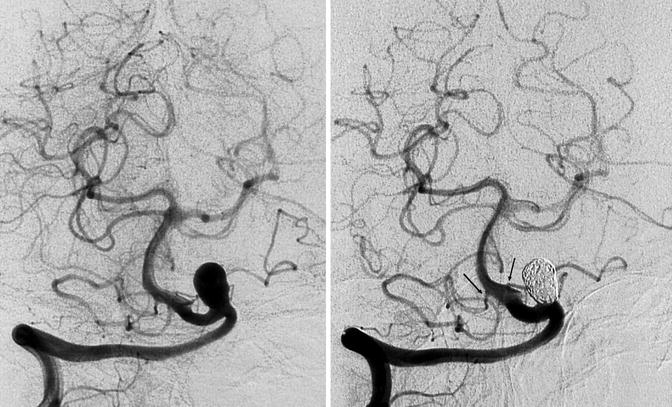

Fig. 11.19
Typical vertebral–posterior inferior cerebellar artery (PICA) junction aneurysm in a patient with SAH, pre- and posttreatment with coils

Fig. 11.20
Large unruptured aneurysm in a young patient. There was a well-developed right vertebral artery and a hypoplastic left vertebral artery. The aneurysm was located at the junction of the vertebral artery with a hypoplastic left PICA. There was a bilateral well-developed anterior inferior cerebellar artery (AICA; arrows), supplying the vascular territory of both PICAs. Right vertebral angiogram pre- and posttreatment with coils
Distal Aneurysms of the Cerebellar Arteries. These are very rare, occurring in fewer than 1 % of all aneurysms (Lubicz et al. 2003; Mitsos et al. 2008). The great majority are located in the PICA (Figs 11.21, 11.22, and 16.9a, b) (Bradac and Bergui 2004; Mitsos et al. 2008), followed by the SCA and AICA (Gacs et al. 1983; Chaloupka et al. 1996; Kurosu et al. 2000; Leonardi et al. 2001; Zager et al. 2002; Menovsky et al. 2002; Gonzales et al. 2004; Peluso et al. 2007; Mitsos et al. 2008; Tokimura et al. 2012). Unlike the majority of intracranial aneurysms, which arise at an arterial branching point, distal cerebellar aneurysm frequently arises directly from the artery where it forms a curve. The course of the cerebellar arteries, especially the PICA, is sometimes characterized by a sharp curve, which could be the cause of the hemodynamic stress on the wall and contribute to the formation of the aneurysm (Lewis et al. 2002; Horiuchi et al. 2003; Mitsos et al. 2008).
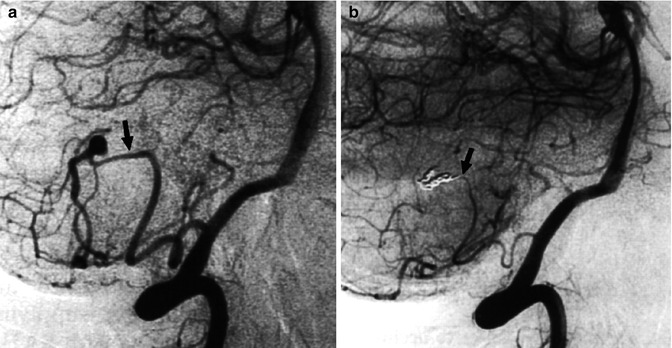
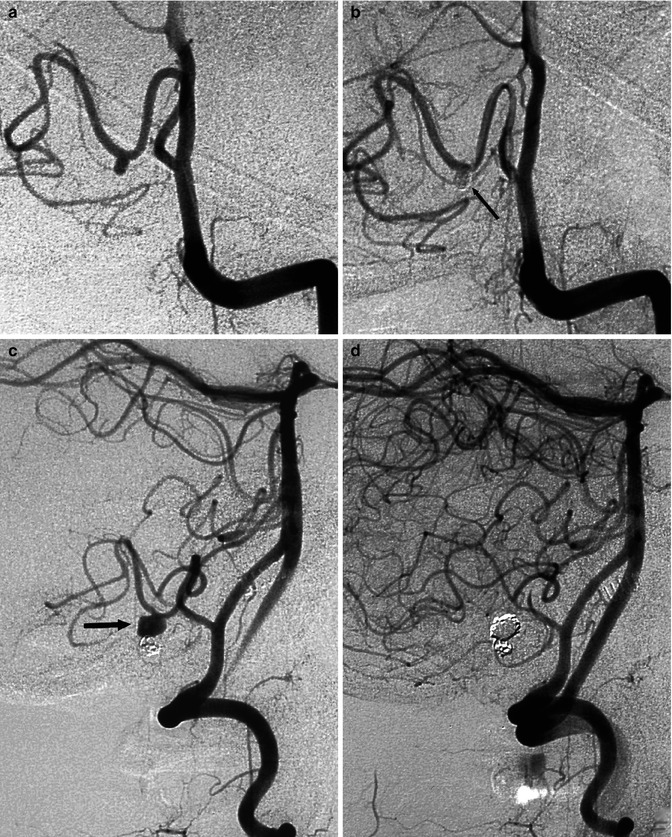

Fig. 11.21
Aneurysm of the supratonsillar segment of the PICA presenting with hemorrhage. (a) Vertebral angiogram showing the aneurysm. Supratonsillar segment (arrow). There is also a dilatation of the retrotonsillar segment of the PICA

Fig. 11.22
Aneurysm of the retromedullary segment of the PICA presenting with hemorrhage. Pretreatment vertebral angiogram (a) presenting the aneurysm and posttreatment angiogram (b) showing its occlusion with coils (arrow). Two months later, the patient was admitted again owing to a new hemorrhage. The angiogram (c) showed regrowth of the aneurysm (arrow), which probably was originally a dissecting aneurysm. Occlusion of the aneurysm and of the parent artery was performed (d). The patient recovered completely
Dissection as possible pathogenesis has increasingly been reported (Lefkowitz et al. 1996; Yamamura et al. 1999; Dinichert et al. 2000; Tawk et al. 2003; Bradac and Bergui 2004; Maimon et al. 2006; Mitsos et al. 2008; Cellerini et al. 2008; Fukushima et al. 2009) (see also Chap. 16).
Flow-dependent aneurysms of the cerebellar arteries related to a more distal AVM, DAVF, or hemangioblastoma are relatively frequent in our experience as well as in the reports of several authors (Hudgins et al. 1983; Kaech et al. 1987; Guzman and Grady 1999; Kaptain et al. 1999; Menovsky et al. 2002; Lewis et al. 2002; Peluso et al. 2007) (Figs. 12.13, 12.14, 12.15, and 12.16).
In aneurysms involving the distal segment of the cerebellar arteries, the occlusion, also of the parent artery, is commonly performed without clinical problems since the perforators for the medulla arise in the proximal segment (see anatomy). Furthermore, the distal cortical branches are revascularized through a frequently rich collateral circulation via anastomoses involving leptomeningeal branches of the cerebellar arteries.
Aneurysms of the Posterior Cerebral Arteries. These rare aneurysms present with a relatively typical location, which is at the P1–P2 passage. More uncommon are aneurysms located along the course of the P2 and at the P2–P3 passage. Such aneurysms are frequently large, sometimes with a fusiform aspect (Li et al. 2007a, b). The etiology is frequently thought to be a dissection (Figs. 11.23, 11.24, and 16.12) (Ciceri et al. 2001; Zhao et al. 2013). This is especially true for such aneurysms in pediatric patients (Laughlin et al. 1997; Lasjaunias et al. 2005; Vilela and Goulao 2006; Bradac et al. 2008a).
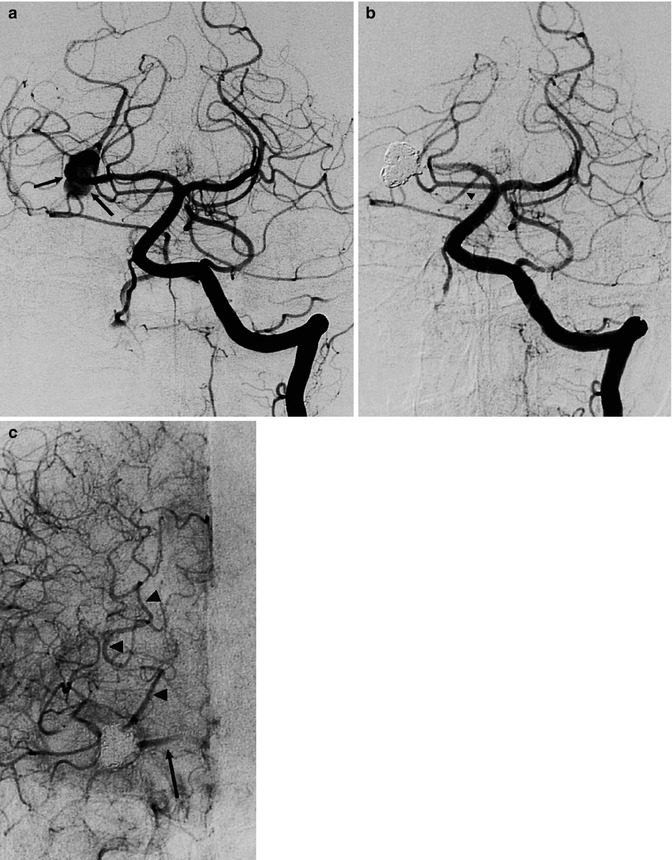

Fig. 11.23




Unruptured irregular, probably dissecting aneurysm involving the P2 segment of the right PCA in a patient presenting with a transient episode of hemianesthesia. Left vertebral angiogram (a) showing the aneurysm (arrows). Posttreatment angiogram (b) with occlusion of the aneurysm and the P2 segment of the PCA. Note the posterior communicating artery (arrowhead). Carotid angiogram (c) with retrograde injection of the posterior cerebral artery up to the aneurysm (arrowheads), through leptomeningeal anastomosis between the MCA and PCA. There is also injection of the proximal P2 (arrow) through the posterior communicating artery. The patient tolerated the treatment well despite a small ischemic lesion in the lateral thalamus owing to involvement of the thalamogeniculate artery, as demonstrated by MRI
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


