Lee A. Fleisher, Joshua A. Beckman
Anesthesia and Noncardiac Surgery in Patients with Heart Disease
Cardiovascular morbidity and mortality represent a special concern in patients with known (or with risk factors for) cardiovascular disease who undergo noncardiac surgery. The cost of perioperative myocardial injury adds substantially to the total health care expenditure, with an average increased length of stay (LOS) of 6.8 days for patients with perioperative myocardial ischemic injury. Perioperative cardiovascular complications not only affect the immediate period but may also the influence outcome over subsequent years. The evidence base for managing patients with cardiovascular disease in the context of noncardiac surgery has grown in recent decades, beginning with identification of those at greatest risk and progressing to randomized trials to identify strategies for reducing perioperative cardiovascular complications. Guidelines provide information for the management of high-risk patients and disseminate best practices. Indeed, over the last decade, mortality rates for all major surgeries have dropped in parallel with implementation of these practices.1 This chapter attempts to distill this information by incorporating guidelines available from the American College of Cardiology Foundation (ACCF)/American Heart Association (AHA) and from the European Society of Cardiology (ESC).2,3 The ACCF/AHA plan an update to this guideline to be published in September 2014.
Additionally, controversy exists regarding research conducted at Erasmus University by Don Poldermans (the DECREASE [Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography] studies), in which an investigative committee found serious shortcomings in the procedure used to record patient consent, submission of publications based on unreliable data, and scientifically inaccurate data collection (http://www.erasmusmc.nl/5663/135857/3675250/3706798/Integrity_report_2012-10.pdf?lang=en. Accessed December 8, 2012). We included these studies for completeness, but their importance with regard to clinical decision making should take into account questions regarding data quality and that the guidelines committee has yet to incorporate this concern into new recommendations.
Assessment of Risk
Much of the contemporary study of perioperative cardiac risk has focused on the development of clinical risk indices. The most widely used index was developed in a study of 4315 patients 50 years or older undergoing elective major noncardiac procedures in a tertiary care teaching hospital. The index includes six independent predictors of complications in a revised cardiac risk index (RCRI): high-risk type of surgery, history of ischemic heart disease, history of congestive heart failure, history of cerebrovascular disease, preoperative treatment with insulin, and preoperative serum creatinine concentration greater than 2.0 mg/dL. Cardiac complication rates rise with an increasing number of these risk factors. Patients are stratified into low, intermediate, or high cardiovascular risk on the basis of having 0, 1 to 2, or 3 or more factors included in the RCRI, respectively. The RCRI has become the standard tool for assessing the probability of perioperative cardiac risk in a given individual and serves to direct the decision to perform cardiovascular testing and implement perioperative management protocols. The RCRI has undergone validation in vascular surgery populations and is used to predict long-term outcome and quality of life, although one group has advocated inclusion of age as a risk factor. Another risk index was developed from the American College of Surgeons 2007 National Surgical Quality Improvement Program data base.4 Of 211,410 patients, perioperative myocardial infarction (MI) or cardiac arrest developed in 1371 (0.65%). Multivariate logistic regression analysis identified five predictors of perioperative MI or cardiac arrest: type of surgery, dependent functional status, abnormal creatinine level, American Society of Anesthesiologists class, and increasing age.
Ischemic Heart Disease
Numerous points of entry lead to evaluation of patients before they undergo noncardiac surgery. Primary physicians or cardiologists may examine such patients. However, many patients are evaluated only immediately before surgery by the surgeon or anesthesiologist. The stress related to noncardiac surgery may raise the heart rate (HR) preoperatively, which is associated with a high incidence of symptomatic and asymptomatic myocardial ischemia. Preoperative clinical evaluation of patients may therefore identify stable or unstable coronary artery disease (CAD). Patients with acute manifestations of CAD such as unstable angina or decompensated heart failure of ischemic origin have a high risk for the development of further decompensation or myocardial necrosis and death during the perioperative period. Such patients clearly warrant further evaluation and medical stabilization. If the noncardiac surgery is truly an emergency, several older case series have shown that intra-aortic balloon bump counterpulsation can provide short-term myocardial protection beyond that afforded by maximal medical therapy, although this measure is seldom used today.
If the patient does not have unstable symptoms, identification of known or symptomatic stable CAD or risk factors for CAD can guide further diagnostic evaluation or changes in perioperative management. In determining the extent of preoperative evaluation it is important to not perform testing unless the results will affect perioperative management. Such changes in management include cancellation of surgery if the risk-benefit ratio is prohibitive, delay of surgery for further medical management, coronary interventions before surgery, use of an intensive care unit (ICU), and changes in monitoring. As discussed later, current data challenge the benefit of preoperative coronary revascularization, findings that can limit the need for extensive testing.
Patients with stable angina represent a continuum from mild angina with extreme exertion to dyspnea with angina after walking up a few stairs. Patients who manifest angina only after strenuous exercise often do not have signs of left ventricular dysfunction and can generally be stabilized with adequate medical therapy—particularly treatment with aspirin, beta-adrenergic–blocking agents, and statins. In contrast, patients with dyspnea on mild exertion would be at high risk for the development of perioperative ventricular dysfunction, myocardial ischemia, and possibly MI. Such patients have a high probability of having extensive CAD and warrant consideration of additional monitoring or cardiovascular testing depending on the surgical procedure, institutional factors, and results of the previous evaluation.
Traditionally, assessment of the coronary risk associated with noncardiac surgery in patients with previous MI was based on the time interval between the MI and surgery. Multiple studies have demonstrated an increased incidence of reinfarction after noncardiac surgery if the previous MI had occurred within 6 months of the operation. Improvements in perioperative care have shortened this time interval, but these criteria have less relevance in the current era of thrombolytic agents, primary percutaneous revascularization, and routine coronary risk stratification after acute MI. Although some patients after a recent MI may continue to have myocardium at risk for subsequent ischemia and infarction, most patients in the United States will have had their critical coronary stenosis evaluated and revascularized when appropriate and should receive maximal medical therapy. The AHA/ACC Task Force on Perioperative Evaluation of the Cardiac Patient Undergoing Noncardiac Surgery has suggested that the highest-risk patients are those within 30 days of MI, during which time plaque and myocardial healing occur. After this period, risk stratification is based on the features of the disease (i.e., those with active ischemia are at highest risk). However, a study using administrative data from California demonstrated that the rate of perioperative cardiac morbidity and mortality remained elevated for at least 60 days after a myocardial infarction.4a
Hypertension
In the 1970s a series of case studies changed the prevailing thought that the use of antihypertensive agents should be discontinued before surgery. The reports suggested that poorly controlled hypertension was associated with untoward hemodynamic responses and that antihypertensive agents should be continued perioperatively. However, several large prospective studies were unable to establish mild to moderate hypertension as an independent predictor of postoperative cardiac complications such as cardiac death, postoperative MI, heart failure, or arrhythmias. The approach to patients with hypertension therefore relies mostly on management strategies from the nonsurgical literature.
A hypertensive crisis in the postoperative period—defined as diastolic blood pressure (BP) higher than 120 mm Hg and clinical evidence of impending or actual end-organ damage—poses a definite risk for MI and cerebrovascular accidents. Diagnostic criteria include papilledema or other evidence of increased intracranial pressure, myocardial ischemia, or acute renal failure. Precipitants of hypertensive crises include preeclampsia or eclampsia, pheochromocytoma, abrupt withdrawal from clonidine therapy before surgery, chronic use of monoamine oxidase inhibitors with or without sympathomimetic drugs, and inadvertent discontinuation of antihypertensive therapy.
Chronic hypertension may predispose patients to perioperative myocardial ischemia because these patients more commonly have concomitant CAD. Recent clinical trials have yielded mixed conclusions regarding the relevance of hypertension to perioperative outcomes. A retrospective evaluation of 2462 patients undergoing vascular surgery showed that adding hypertension to a risk prediction model improved its prognostic ability.5 In contrast, in the POISE (PeriOperative ISchemic Evaluation) trial of beta-adrenergic blockade, 62% of the 8351 subjects had chronic hypertension, but it was not a predictor of postoperative stroke or death.6 Thus the preoperative BP of hypertensive patients with known peripheral and coronary vascular disease should be monitored and controlled.
Whether patients with mild to moderate hypertension should be considered at greater risk for perioperative myocardial ischemia remains uncertain. Surgery generally need not be postponed or canceled in otherwise uncomplicated patients with mild to moderate hypertension. Antihypertensive medications should be continued perioperatively, and BP should be maintained near preoperative levels to reduce the risk for myocardial ischemia. In patients with more severe hypertension, such as a diastolic BP higher than 110 mm Hg, the potential benefits of delaying surgery to optimize antihypertensive medications should be weighed against the risk associated with delaying the surgical procedure. With rapidly acting intravenous agents, BP can usually be controlled within several hours. As cited in the ACCF/AHA guidelines (referred to hereafter as the guidelines), Weksler and colleagues studied 989 chronically hypertensive patients about to undergo surgery for noncardiac reasons with a diastolic BP between 110 and 130 mm Hg and no previous MI, unstable or severe angina pectoris, renal failure, pregnancy-induced hypertension, left ventricular hypertrophy, previous coronary revascularization, aortic stenosis, preoperative dysrhythmias, conduction defects, or stroke. The control group had their surgery postponed and remained in the hospital for control of BP, and the study patients received 10 mg of nifedipine delivered intranasally.2 The study did not find statistically significant differences in postoperative complications, thus suggesting that this subset of patients without manifest cardiovascular comorbid conditions can proceed with surgery despite elevated BP on the day of surgery.
Heart Failure
Heart failure is associated with perioperative cardiac morbidity after noncardiac surgery in virtually all studies. Goldman and colleagues, as cited in the guidelines, identified a third heart sound or signs of heart failure as portending the highest perioperative risk. In patients with signs or symptoms of heart failure who are scheduled for noncardiac surgery, causes of the heart failure require characterization.2 The preoperative evaluation should aim to identify the underlying coronary, myocardial, and/or valvular heart disease and assess the severity of the systolic and diastolic dysfunction. Hammill and associates used Medicare claims data to evaluate short-term outcomes in patients with heart failure, CAD, or neither who underwent major noncardiac surgery.7 Elderly patients with heart failure who undergo major surgical procedures were found to have substantially higher risk for operative mortality and hospital readmission than were other patients, including those with CAD, admitted for the same procedures. Use of codes from the International Classification of Diseases, ninth edition, for patient identification does not permit differentiation between heart failure with preserved or impaired left ventricular function, and thus the preoperative evaluation can influence preoperative management beyond just a high-risk classification; in particular, this assessment may influence perioperative fluid and vasopressor management.
Treatment of decompensated hypertrophic cardiomyopathy differs from that of dilated cardiomyopathy, and thus the preoperative evaluation can influence perioperative management; in particular, this assessment may influence perioperative fluid and vasopressor management. Ischemic cardiomyopathy is of greatest concern because the patient has substantial risk for the development of further ischemia, which can lead to myocardial necrosis and potentially a downward spiral. A pulmonary artery catheter or intraoperative transesophageal echocardiography (TEE) may be helpful in such patients.
Obstructed hypertrophic cardiomyopathy was formerly regarded as a high-risk condition associated with high perioperative morbidity. A retrospective review of perioperative care in 35 patients, however, suggested that the risk related to general anesthesia and major noncardiac surgery is low in such patients. This study also suggested spinal anesthesia to be a relative contraindication in view of the sensitivity of cardiac output to preload in this condition. Haering and colleagues, as cited in the guidelines, studied 77 patients with asymmetric septal hypertrophy identified retrospectively from a large data base.2 Forty percent of the patients had one or more adverse perioperative cardiac events, including one patient who had an MI and ventricular tachycardia that required emergency cardioversion. Most of the events consisted of perioperative congestive heart failure, and no perioperative deaths occurred. Unlike the finding in the original cohort of patients, the type of anesthesia was not an independent risk factor. Important independent risk factors for an adverse outcome (as seen generally) included major surgery and increasing duration of surgery.
Valvular Heart Disease (See also Chapter 63)
Aortic stenosis places patients at increased risk. Critical stenosis is associated with the highest risk for cardiac decompensation in patients undergoing elective noncardiac surgery. As cited in the guidelines, Kertai reported a substantially higher rate of perioperative complications in patients with severe aortic stenosis than in those with moderate aortic stenosis (31% [5/16] versus 11% [10/92]).2,8 The presence of any of the classic triad of angina, syncope, and heart failure in a patient with aortic stenosis should prompt further evaluation and potential interventions (usually valve replacement). Many patients with severe or critical aortic stenosis are asymptomatic. Preoperative patients with aortic systolic murmurs warrant a careful history and physical examination—and often further evaluation. Several recent case series of patients with critical aortic stenosis have demonstrated that when necessary, noncardiac surgery can be performed with acceptable risk. For the most part, these series have included patients with few or no symptoms but a valve area smaller than 0.5 cm2. Aortic valvuloplasty is an alternative option for some patients. Although the long-term outcome of patients who undergo aortic balloon valvuloplasty is generally poor, primarily because of restenosis, this procedure can provide temporary benefit before noncardiac surgery in patients who cannot undergo valve replacement in the short term. The substantial risk for procedure-related morbidity and mortality requires careful consideration before recommending this strategy to lower the risk imposed by noncardiac surgery.
Mitral valve disease is associated with a lower risk for perioperative complications than aortic stenosis is, although occult mitral stenosis secondary to rheumatic heart disease sometimes occurs and can lead to severe left-sided heart failure in patients with tachycardia (e.g., uncontrolled atrial fibrillation) and/or volume loading. In contrast to aortic valvuloplasty, mitral valve balloon valvuloplasty often yields both short- and long-term benefit, especially in younger patients with predominantly mitral stenosis but without severe mitral valve leaflet thickening or significant subvalvular fibrosis and calcification.
In perioperative patients with a functioning prosthetic heart valve, antibiotic prophylaxis and anticoagulation are major issues. All patients with prosthetic valves who undergo procedures that can cause transient bacteremia should receive prophylaxis. In patients with prosthetic valves the risk for increased bleeding during a procedure while receiving antithrombotic therapy must be weighed against the increased risk for thromboembolism caused by stopping the therapy. Common practice in patients undergoing noncardiac surgery with a mechanical prosthetic valve in place is cessation of oral anticoagulants 3 days before surgery. This allows the international normalized ratio to fall to less than 1.5 times normal; oral anticoagulants can then be resumed on postoperative day 1. An alternative approach in patients at high risk for thromboembolism is conversion to heparin during the perioperative period, which can then be discontinued 4 to 6 hours before surgery and resumed shortly thereafter. A multicenter, single-arm cohort study of 224 high-risk patients (prosthetic valves, atrial fibrillation, and a major risk factor) investigated the use of low-molecular-weight heparin (LMWH) as a preoperative bridge to warfarin anticoagulation in which warfarin was withheld for 5 days and LMWH was given 3 days preoperatively and at least 4 days postoperatively. The overall rate of thromboembolism was 3.6%, and the overall rate of cardioembolism was 0.9%. Major bleeding was seen in 6.7% of subjects, although only 8 of 15 episodes occurred during the administration of LMWH.9 LMWH is cost-effective because it helps reduce the duration of the hospital stay, but two studies have shown a residual anticoagulation effect in as many as two thirds of patients.10,11
Many current prosthetic valves have a lower risk for valve thrombosis than the older ball-in-cage valves do, so the risk associated with heparin may outweigh its benefit in the perioperative setting. According to the AHA/ACCF guidelines, heparin can usually be reserved for high-risk patients. High risk is defined by the presence of a mechanical mitral or tricuspid valve or a mechanical aortic valve and by certain risk factors, including atrial fibrillation, previous thromboembolism, hypercoagulable condition, older-generation mechanical valves, an ejection fraction lower than 30%, or more than one mechanical valve.12 Subcutaneous LMWH or unfractionated heparin offers an alternative outpatient approach but has received only a tentative recommendation. Discussion between the surgeon and cardiologist regarding optimal perioperative management is critical.
Congenital Heart Disease in Adults (See also Chapter 62)
Congenital heart disease afflicts 500,000 to 1 million adults in the United States. The nature of both the underlying anatomy and any anatomic correction affects the perioperative plan and incidence of complications, which include infection, bleeding, hypoxemia, hypotension, and paradoxical embolization. A major concern in patients with congenital heart disease is the presence of pulmonary hypertension and Eisenmenger syndrome. Regional anesthesia has traditionally been avoided in these patients because of the potential for sympathetic blockade and worsening of the right-to-left shunt. However, a review of the published literature incorporating 103 cases found that overall perioperative mortality was 14%; patients receiving regional anesthesia had a mortality of 5%, whereas those receiving general anesthesia had a mortality of 18%. The authors concluded that most deaths probably resulted from the surgical procedure and the disease rather than from anesthesia. Although perioperative and peripartum mortality was high, many anesthetic agents and techniques had been used with success. Patients with congenital heart disease are at risk for infective endocarditis and should receive antibiotic prophylaxis. A recent review has discussed the anesthetic management of these patients in detail.8
Arrhythmias (See Chapters 34 Through 39)
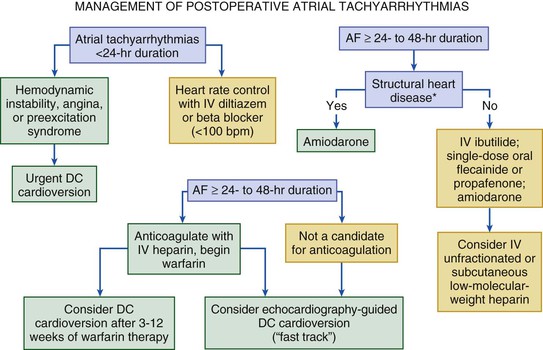
Cardiac arrhythmias commonly occur in the perioperative period, particularly in older adults or patients undergoing thoracic surgery. Predisposing factors include previous arrhythmias, underlying heart disease, hypertension, perioperative pain (e.g., hip fractures), severe anxiety, and other situations that heighten adrenergic tone. In a prospective study of 4181 patients 50 years or older, supraventricular arrhythmia occurred in 2% during surgery and in 6.1% after surgery. Perioperative atrial fibrillation raises several concerns, including the incidence of stroke (see Chapter 38). Winkel and colleagues evaluated 317 patients without atrial fibrillation who were undergoing major vascular surgery to determine the incidence of new-onset atrial fibrillation and its association with adverse cardiovascular outcomes. They reported an incidence of 4.7% and more than a sixfold increase in cardiovascular death, MI, unstable angina, and stroke in the first 30 days and a fourfold increase over the next 12 months.13 Early treatment to restore sinus rhythm or control the ventricular response and initiate anticoagulation is therefore indicated. Prophylactic use of intravenous diltiazem in randomized, placebo-controlled trials of patients undergoing high-risk thoracic surgery was found to reduce the incidence of clinically significant atrial arrhythmias.2 Balser and colleagues studied 64 cases of postoperative supraventricular tachyarrhythmia. After the administration of adenosine, patients who remained in supraventricular tachyarrhythmia were prospectively randomly assigned to receive either intravenous diltiazem or intravenous esmolol for control of the ventricular rate; intravenous esmolol produced a more rapid (2-hour) conversion to sinus rhythm than did intravenous diltiazem.14 Figure 80-1 presents an algorithm for the management of atrial fibrillation.
Although older studies identified ventricular arrhythmias as a risk factor for perioperative morbidity, recent studies have not confirmed this finding. O’Kelly, as cited in the guidelines, studied a consecutive sample of 230 male patients with known CAD or at high risk for CAD who underwent major noncardiac surgical procedures.2 Preoperative arrhythmias were associated with intraoperative and postoperative arrhythmias, but nonfatal MI and cardiac death were not substantially more frequent in those with previous perioperative arrhythmias. Amar and coworkers studied 412 patients undergoing major thoracic surgery and determined that even though the incidence of nonsustained ventricular tachycardia was 15%, it was not associated with a poor outcome.15 Despite this finding, the presence of an arrhythmia in the preoperative setting should provoke a search for underlying cardiopulmonary disease, ongoing myocardial ischemia or infarction, drug toxicity, or electrolyte or metabolic derangements.
Conduction abnormalities can increase perioperative risk and may require placement of a temporary or permanent pacemaker. On the other hand, patients with intraventricular conduction delays, even in the presence of a left or right bundle branch block but without a history of advanced heart block or symptoms, rarely progress to complete heart block perioperatively. The availability of transthoracic pacing units has decreased the need for temporary transvenous pacemakers.
The Decision to Undergo Diagnostic Testing
The ACCF/AHA and ESC both proposed algorithms based on the available evidence and incorporated the class of recommendations and level of evidence into each step (Fig. 80-2). Current algorithms use a stepwise bayesian strategy that relies on assessment of clinical markers, previous coronary evaluation and treatment, functional capacity, and surgery-specific risk (as outlined later). Successful use of the algorithms requires an appreciation of the different levels of risk attributable to certain clinical circumstances, levels of functional capacity, types of surgery, and how the information from any diagnostic testing will influence perioperative management.
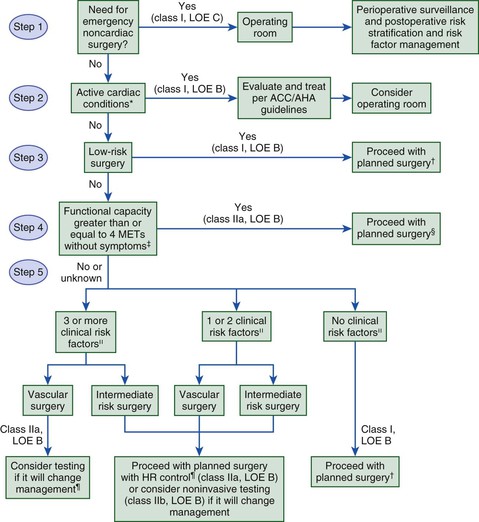
Multiple studies have attempted to identify clinical risk markers for perioperative cardiovascular morbidity and mortality. As described earlier, patients with unstable coronary syndromes and severe valvular disease have active cardiac conditions. Patients with known stable CAD are at intermediate risk. Other clinical risk factors in the RCRI make up the remainder of the predictors of intermediate risk (history of congestive heart failure, history of cerebrovascular disease, preoperative treatment with insulin, and preoperative serum creatinine concentration >2.0 mg/dL). Cardiovascular disease also has clinical risk markers that have been classified as “low-risk factors,” each of which is associated with variable levels of perioperative risk. Table 80-1 shows the classification of perioperative clinical risk markers for the purpose of assessing the need for further testing.
TABLE 80-1
Active Cardiac Conditions for Which Patients Should Undergo Evaluation and Treatment Before Noncardiac Surgery (Class I; Level of Evidence: B)
| CONDITION | EXAMPLES |
| Unstable coronary syndromes | |
| Decompensated HF (NYHA functional class IV; worsening or new-onset HF) | |
| Significant arrhythmias | |
| Severe valvular disease |
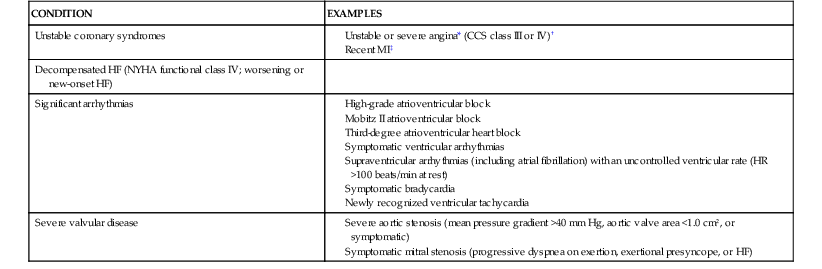
* According to Campeau L, Enjalbert M, Lesperance J, et al: Atherosclerosis and late closure of aortocoronary saphenous vein grafts: Sequential angiographic studies at 2 weeks, 1 year, 5 to 7 years, and 10 to 12 years after surgery. Circulation 68(Suppl II):1, 1983.
† May include “stable” angina in patients who are unusually sedentary.
‡ The American College of Cardiology National Database Library defines recent MI as more than 7 days but 1 month or less (within 30 days).
CCS = Canadian Cardiovascular Society; HF = heart failure; MI = myocardial infarction; NYHA = New York Heart Association.
From Fleisher LA, Beckman JA, Brown KA, et al: 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 120:e169, 2009.
As described with regard to the anginal pattern, exercise tolerance is one of the strongest determinants of perioperative risk and the need for invasive monitoring. In one study of outpatients referred for evaluation before major noncardiac procedures, patients were asked to estimate the number of blocks that they could walk and flights of stairs that they could climb without experiencing cardiac symptoms. Patients who could not walk four blocks and could not climb two flights of stairs were considered to have poor exercise tolerance and had twice as many perioperative cardiovascular complications as did those with better functional status. The likelihood of a serious complication related inversely to the number of blocks that could be walked or flights of stairs that could be climbed. Several scales based on activities of daily living have been proposed to assess exercise tolerance. The current guidelines advocate one such scale (the Duke Activity Scale Index) (Table 80-2). (See also Chapters 47 and 79.)
TABLE 80-2
Estimated Energy Requirements for Various Activities
| CAN YOU … | |
| 1 MET | |
| 4 METs | |
| >10 METs | Participate in strenuous sports such as swimming, singles tennis, football, basketball, or skiing? |

kph = kilometers per hour; MET = metabolic equivalent; mph = miles per hour.
Modified from Hlatky MA, Boineau RE, Higgenbotham MB, et al: A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol 64:65, 1989. Copyright 1989, with permission from Elsevier; modified from Fleisher LA, Beckman JA, Brown KA, et al: 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 120:e169, 2009.
The type of surgical procedure has a significant impact on perioperative risk and the amount of preparation required to perform anesthesia safely. For surgical procedures not associated with significant stress or a high incidence of perioperative myocardial ischemia or morbidity, the cost of the evaluation often exceeds any benefit from the information gained by preoperative assessment. Outpatient procedures, for example, cause little morbidity and mortality; in such patients, cardiovascular status rarely changes perioperative management unless the patient has unstable angina or overt congestive heart failure. In fact, 30-day mortality after outpatient surgery may actually be lower than that expected if the patient did not undergo surgery. In contrast, open surgery for vascular disease entails a high risk for morbidity and the potential for ischemia. Intra-abdominal, thoracic, and orthopedic procedures are associated with intermediate risk (Table 80-3). Endovascular procedures fall into this intermediate-risk category on the basis of their associated perioperative morbidity and mortality, although long-term survival appears to be similar to that in patients who undergo open procedures.
TABLE 80-3
Cardiac Risk* Stratification for Noncardiac Surgical Procedures
| RISK STRATIFICATION | EXAMPLES OF PROCEDURES |
| High (reported cardiac risk often >5%) | |
| Intermediate (reported cardiac risk generally 1-5%) | |
| Low† (reported cardiac risk generally <1%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree