Airway disease
Asthma, cystic fibrosis, interstitial emphysema
Postinfectious
Pneumocystis jirovecii, tuberculosis, lung abscess, necrotizing pneumonia, HIV, parasites
Connective tissue diseases
Marfan syndrome, Ehlers–Danlos syndrome, juvenile idiopathic arthritis, systemic lupus erythematosus, dermatomyositis, Birt–Hogg–Dube syndrome
Oncological diseases
Lymphoma, pulmonary metastasis
Congenital malformations
Cystic adenomatoid malformation
Congenital lobar emphysema
Foreign body aspiration
Catamenial pneumothorax
Related to menstrual cycle
When considering pediatric thoracic trauma, pneumothorax may occur in up to a third of patients, most of them associated with extrathoracic and intrathoracic injuries, but one third may present only pneumothorax.
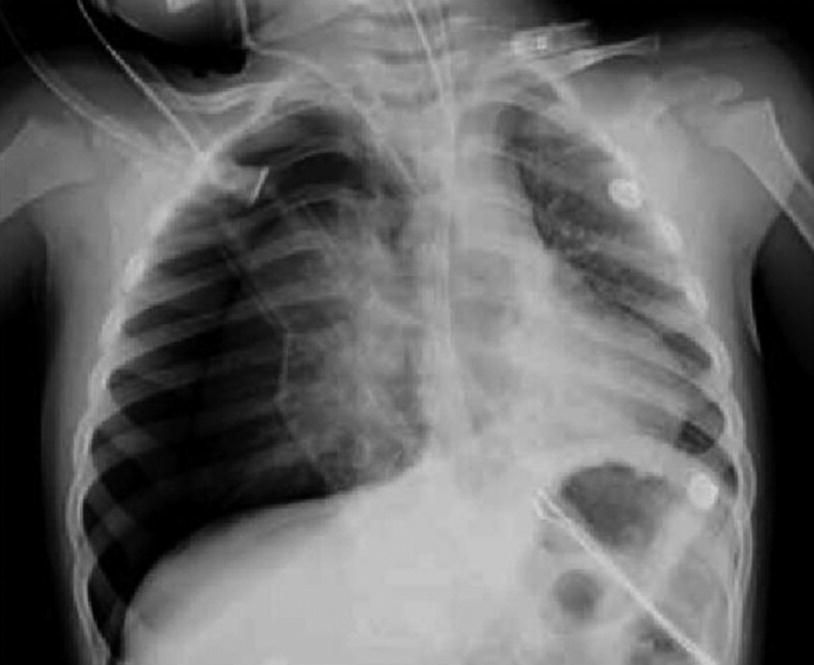
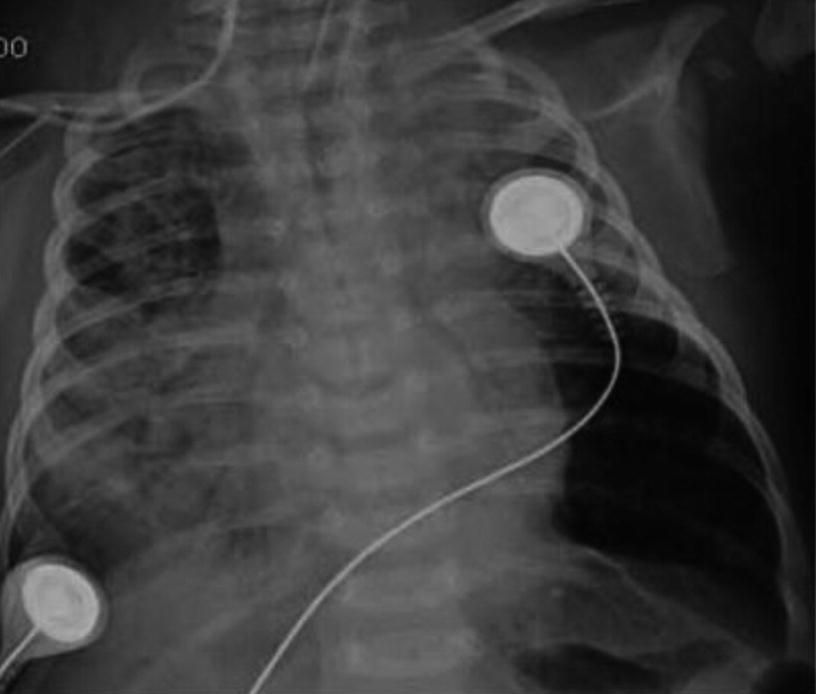
Iatrogenic Pneumothorax. Anteroposterior chest X-ray of a 2-year-old patient on mechanical ventilation due to convulsive status. It shows right-sided pneumothorax secondary to insertion of a central venous catheter into the internal jugular vein on the same side
Etiology and Pathophysiology
The increase in transpulmonary pressure is the mechanism described in the appearance of spontaneous pneumothorax. This causes alveolar distension, and if the gradient is high enough, alveolar rupture. It can occur secondary to increased work of breathing, a Valsalva maneuver, positive pressure ventilation, and airway obstruction that causes an obstruction ball valve effect, among others.
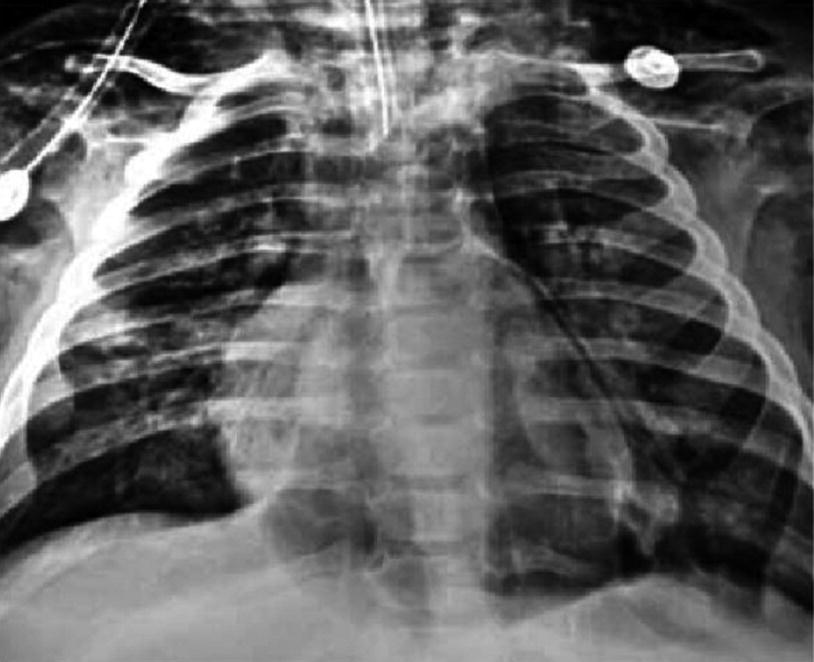
Postinfectious Pneumothorax. Anteroposterior chest x-ray of an 8-month-old patient with severe pneumonia due to adenovirus. It shows pneumothorax, pneumomediastinum, pneumopericardium, and subcutaneous emphysema secondary to mechanical ventilation
Spontaneous pneumothorax seems to occur due to changes in the connective tissue that predispose air leaks from the airways into the pleural space. The mechanisms proposed for both primary and secondary pneumothorax are multiple. Frequently, the existence of subpleural bullae, or bullas, has been associated with the production of primary pneumothorax. In studies performed with computerized axial tomography scans, bullas have been detected in between 28% and 100% of the cases that developed a primary pneumothorax and between 70% and 95% in patients who required video-assisted thoracoscopy surgery (VATS) due to this pathology. In newborns, high transpulmonary pressures during the first breaths contribute to the appearance of primary pneumothorax.
The role of tobacco, both in the development of primary and secondary spontaneous pneumothorax , has also been widely analyzed, especially in adults. Prevalence of smoking in children with primary pneumothorax is between 11% and 14%, while in adults is between 24% and 88%. This supports the theory between time exposure to tobacco and changes in connective tissue and/or chronic bronchiolitis that are observed in adult patients and predisposition to pneumothorax.
A familial form of primary spontaneous pneumothorax has been described, related to a mutation in the folliculin gene, located on chromosome 17, which plays a role in the definition of cell shape, size, and movement. This condition has been associated with the Birt–Hogg–Dube syndrome, in which there is a predisposition to the formation of multiple lung cysts that lead to the development of primary spontaneous pneumothorax.
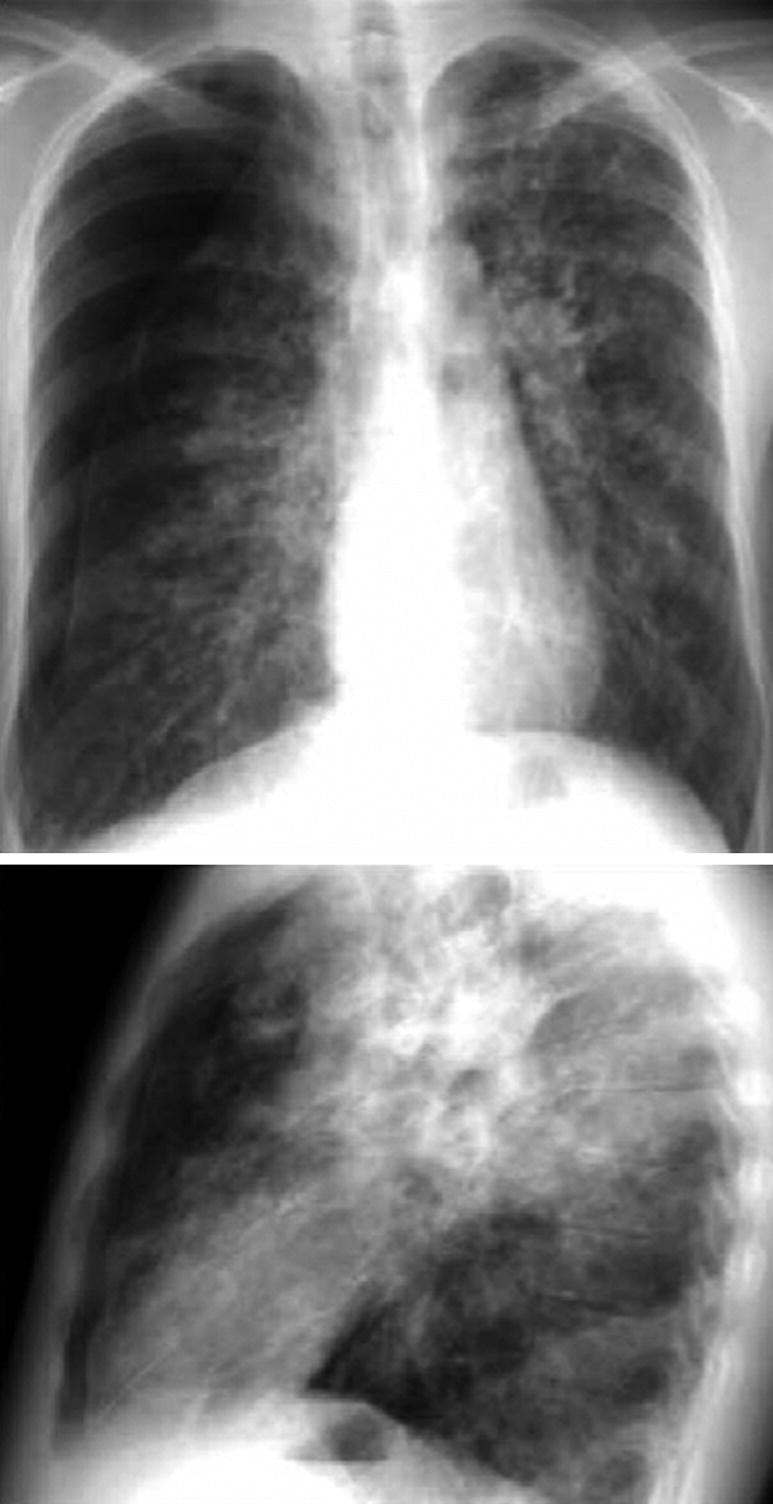
Pneumothorax in Chronic Disease. Anteroposterior and lateral chest X-ray of a 14-year-old patient with cystic fibrosis and advanced lung disease. It shows lateral and anterior pneumothorax on the right side
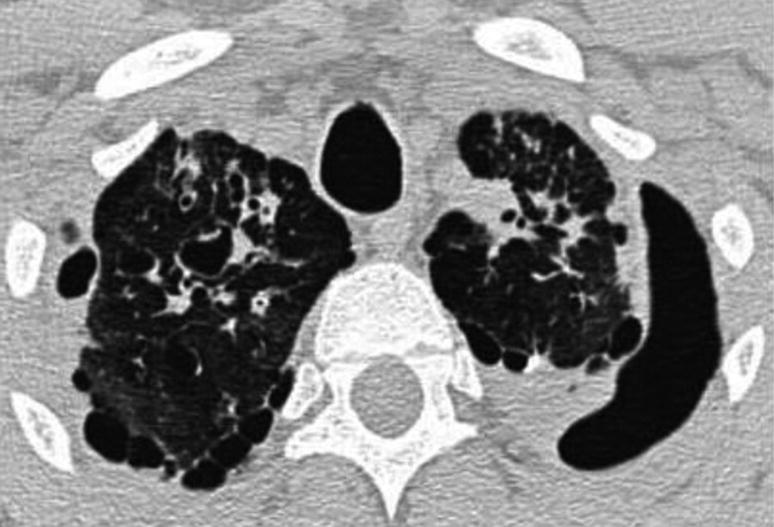
Pneumothorax in Chronic Disease. Chest CAT scan in a patient with cystic fibrosis. It shows left apical pneumothorax and multiple bilateral subpleural bullas
In newborns, the most common causes of secondary spontaneous pneumothorax are meconium aspiration syndrome (MAS) and hyaline membrane disease (HMD).
Clinical Manifestations
Symptoms and signs of pneumothorax vary according to the patient’s age, level of lung collapse and previous lung volumes. Small pneumothoraxes can be asymptomatic and show normal vital signs. Spontaneous pneumothorax occurs mostly at rest, but it may be caused by situations that increase intrathoracic pressure through the Valsalva maneuver (such as lifting objects or stretching).
Acute chest pain and dyspnea are the most frequent symptoms in pediatric spontaneous pneumothorax. Even without treatment, the symptoms resolve in 1–3 days; however, pneumothorax persists in most patients. Physical examination findings depend on the size of the pneumothorax. Small ones may not be detected clinically. Clinical signs include decreased pulmonary sounds, dyspnea, tachycardia, hyperresonance on percussion, and decreased vocal fremitus. In a minority of patients it can present itself as tension pneumothorax, with respiratory distress, hypoxemia, tachycardia, and arterial hypotension. The latter is a respiratory emergency and requires immediate intervention.
Diagnostic Approach

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


