Anatomical and Functional Mitral Valve Abnormalities in the Pediatric Population
Andrew Mackie
Jeffrey Smallhorn
Introduction
This chapter deals with mitral valve abnormalities that are encountered in the pediatric population. We include abnormalities of the morphologic mitral valve in corrected transposition, but exclude abnormalities in the setting of a univentricular atrioventricular connection, left side atrioventricular valve in atrioventricular septal defect, and mitral valve abnormalities in rheumatic heart disease. As well we exclude hearts with hypoplastic left heart syndrome, as these patients follow a single ventricle pathway. Although some mitral valve abnormalities occur in isolation, many are part of a spectrum of left-sided congenital abnormalities. If missed, these associated lesions often have profound implications with regard to patient outcome and hence a high index of suspicion is essential when evaluating a patient with abnormalities of the mitral valve.
Although clinical signs and symptoms are important, noninvasive imaging, in particular echocardiography, is the current cornerstone to detailed anatomical and physiologic assessment and subsequent management implications of congenital mitral valve disease. Three-dimensional echocardiography provides novel anatomical and functional information above and beyond two-dimensional echocardiography and will be emphasized throughout this chapter.
Mitral Valve Development
Following rightward looping of the heart tube the atrioventricular junction becomes prominent around the 25th day of gestation (1,2). The future left ventricle supports the larger part of the circumference of the atrioventricular canal by the end of the fifth week. The atrioventricular canal is occupied by the inferior and superior endocardial cushions which fuse during the 6th week, producing separate right and left atrioventricular junctions. Parts of these fused cushions remain on the left side of the muscular septum, forming the scaffold of the anterior or aortic leaflet of the mitral valve.
The normal mitral valve proceeds when the aorta becomes committed to the left ventricle, permitting the development of fibrous continuity between one of the leaflets of the mitral valve and two of the leaflets of the aortic valve. Initially, there is a cleft at the site of fusion of the superior and inferior cushions within the left ventricle. The mural leaflet, or posterior leaflet is formed by protrusion and growth of a sheet of atrioventricular myocardium into the ventricular lumen, with subsequent formation of valvar mesenchyme on its surface (3). The myocardial layer is then removed by apoptosis. The aortic leaflet of the mitral valve develops from mesenchyme of the superior and inferior artioventricular cushions and is never attached to, or supported, by the myocardium apart from its attachment to the papillary muscles. The inferior quadrants of the left atrioventricular junction expand involving growth of the parietal wall of the left ventricle, with comparable growth of the lateral cushion. This results in the lateral cushion occupying two-thirds of the circumference of the developing mitral valvar orifice; hence the larger mural or posterior leaflet that is seen during clinical or pathologic assessment. The papillary muscles develop from compacting columns in the trabecular layer of the ventricular muscle. Importantly, the mitral valve develops and functions as a unit, which has profound implications during life. With the advent of three-dimensional echocardiography we are moving away from thinking of the mitral valve as separate units, but many that interact with each other, as well as the left ventricular myocardium.
Reciprocal signaling between the endocardial and myocardial cell layers in the cushion is mediated in part by members of the TGF-β family, and induces a transformation of endocardial cells into mesenchymal cells. Sox9 is activated when endocardial cells undergo mesenchymal transformation and Sox9 deficient mesenchymal cells fail to express ErbB3, which is required for proliferation of the cells within the endocardial cushions. The mesenchymal cells migrate into the cushions, and differentiate into the fibrous tissue of the valves. Several genes play a role in formation of the valvar leaflets, with signalling and downstream activation dependent on the NF-AT family of transcription factors. Absence of any of these genes results in fatal defects of valvar formation (4,5,6).
Incidence of Mitral Valve Abnormalites
Congenital mitral valve abnormalites are rare. Congenital mitral stenosis accounts for 0.6% of congenital heart disease (CHD) in postmortem studies and 0.21% to 0.42% when examined in clinical series (7). Isolated congenital mitral valve regurgitation is even rarer and indeed if encountered should be considered as potentially being secondary to another process such as rheumatic fever or in the younger patient, anomalous left coronary artery from the pulmonary artery (8). The male to female ratio of congenital mitral stenosis is of the order of 1.5:1. It is important to consider associated mitral valve pathology in any child presenting with a left-sided obstructive
lesion, such as coarctation of the aorta or aortic valve stenosis. As well, mitral stenosis may be associated with many other congenital lesions. Therefore assessment of mitral valve morphology and function should be part of the evaluation of all patients with CHD.
lesion, such as coarctation of the aorta or aortic valve stenosis. As well, mitral stenosis may be associated with many other congenital lesions. Therefore assessment of mitral valve morphology and function should be part of the evaluation of all patients with CHD.
Mitral Valve Morphology and Function
Although the morphologist has previously been the master in this area, that role has now fallen to the echocardiographer, who can evaluate both morphology and function.
Mitral Annulus
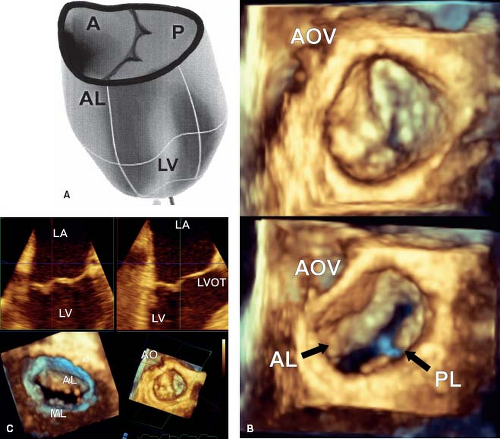
The mitral annulus is mostly a muscular structure that is attached to the left atrium by fibrous connections. Three-dimensional imaging has demonstrated that it is saddle-shaped (9), with two high and two low points (Fig. 43.1A). The high points are at the site of aortic-mitral continuity and at the posterior aspect of the valve, while the two low points are along the lines of the commissure between the aortic and mural leaflets. The region of the mitral annulus that is not muscular is the site of aortic-mitral fibrous continuity. This is an important component, since changes in the mitral annulus during the cardiac cycle
are heterogeneous, such that the outlet maintains maximum dimension during contraction to prevent any impediment to flow (10,11).
are heterogeneous, such that the outlet maintains maximum dimension during contraction to prevent any impediment to flow (10,11).
The saddle shape of the mitral annulus has significant implications with regard to leaflet stress. If the annulus is more planar in shape, this increases leaflet stress, which has negative implications with regard to mitral valve function (12,13). This in part explains why standard mitral valve rings fail, as they flatten the mitral valve annulus (and do not address the underlying pathology).
Starting at end diastole and with atrial contraction the annulus begins to contract, attaining its smallest area just before ventricular systole, presumably to aid in leaflet closure. During midventricular systole there is a dominant inward motion of the annulus in an anterior–posterior direction. This helps maintain an oval shape just when the orifice area is at its greatest, with the added advantage of helping to keep the leaflets together. During ventricular contraction the annulus descends with a gradual increase in annular area, height, and bending angle. This reaches its maximum at end systole and during the period of isovolumic relaxation. With rapid ventricular filling there is a rapid reduction in mitral valve area (14,15,16,17), more so in children which may be related to their superior ventricular relaxation (14,15,16,17). These changes are most likely related to the torsional deformation of the left ventricle from base to apex, demonstrating the importance of thinking of the mitral valve as one unit, with important interactions with the left ventricle.
Mitral Valve Leaflets and Supporting Apparatus
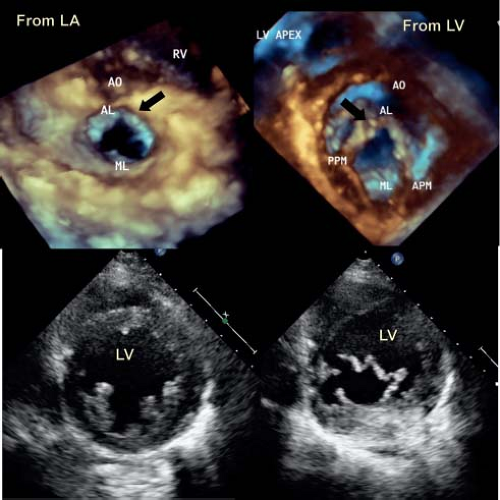
The normal mitral valve has two leaflets, the anterior or aortic and the posterior or mural (Fig. 43.1B). The anterior leaflet is in fibrous continuity with the noncoronary cusp of the aortic valve and hinges from the annulus with support at two commissures by the anterior and posterior papillary muscles (1,18). The anterior leaflet has been arbitarily divided into three components, A1 to A3, which is a useful description for both the echocardiographer and the surgeon. A1 sits adjacent to the anterior commissure while A3 sits adjacent to the posterior commissure. The mural or posterior leaflet is anchored along the parietal part of the left atrioventricular junction. As with its anterior counterpart, this is divided into three components, P1 to P3. Like the anterior leaflet, the posterior leaflet is supported by both papillary muscles. The posterior leaflet frequently has a series of scallops which are supported by chordal structures (Fig. 43.1C).
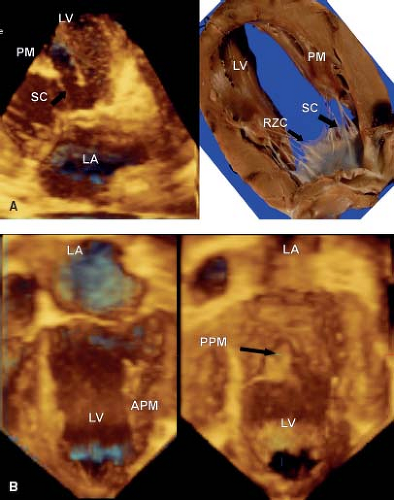
Three-dimensional echocardiography has provided the ability to assess not only the morphology, but the dynamic changes that are seen in the mitral valve. This technique emphasizes that the leaflets are not flat, but consist of a series of undulations which are in part related to the chordal attachments (13) (see Fig. 43.1A). There are several chordal support mechanisms that can be appreciated in pathologic specimens (19), as well as by real-time three-dimensional echocardiography. The strut chordae insert into the undersurface of the anterior leaflet and in part result in the appearance of a series of peaks and valleys in the anterior leaflet when seen in real-time from the left atrial view (Fig. 43.2A). These chordae run onto the belly of the leaflet, whereas the rough zone chordae insert into the tip of the anterior leaflet (Fig. 43.2A). These latter chordae are responsible for preventing leaflet prolapse. The strut chordae are important in situations of ischemic mitral valve regurgitation where they are displaced, resulting in leaflet tethering and regurgitation (20). Next, there are commissural chords, which by definition, support the two mitral valve commissures, while the cleft chordae support the scallops of the posterior or mural leaflet. When viewed
from above by three-dimensional echocardiography during systole, the leaflets appear to be a series of peaks and valleys, the coaptation of which prevents regurgitation (13). The zones of coaptation of the leaflets roll over each other, providing the maximum area of contact, which maintains valve competence. It is when pathologic processes disturb this relationship that regurgitation is seen. Pathologic specimens and surgical inspection, along with saline testing of the valve provide a suboptimal assessment of the functional nature of the mitral valve; three-dimensional echocardiography overcomes this limitation (21).
from above by three-dimensional echocardiography during systole, the leaflets appear to be a series of peaks and valleys, the coaptation of which prevents regurgitation (13). The zones of coaptation of the leaflets roll over each other, providing the maximum area of contact, which maintains valve competence. It is when pathologic processes disturb this relationship that regurgitation is seen. Pathologic specimens and surgical inspection, along with saline testing of the valve provide a suboptimal assessment of the functional nature of the mitral valve; three-dimensional echocardiography overcomes this limitation (21).
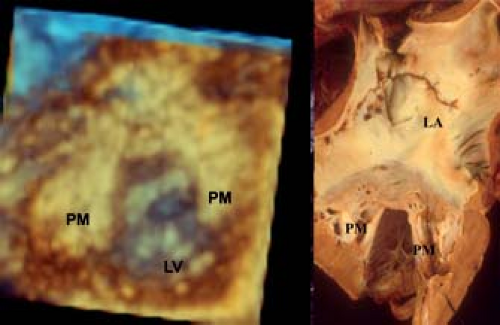
The two papillary muscles are evenly placed such that they maintain constant tension on the leaflets throughout systole (Fig. 43.2B). The angle between the papillary muscle tips and the mitral annulus is about 70 to 80 degrees, as determined by three-dimensional echocardiography. This is achieved by the ability to map the coordinates of all of the components of the mitral valve througout the cardiac cylce and relate them to each other. This papillary muscle angle does not change throughout the cardiac cycle, despite the influence of ventricular contraction and torsion of the left ventricle. The chordae fan out as they insert into the leaflets, but the direction of pull on the leaflets is relatively vertical, avoiding tension on the leaflets. The papillary muscle morpholgy is also variable, in particular with regard to the number of heads. The papillary muscles are derived from left ventricular myocardium and blend in with the wall of the left ventricle, playing an important role in maintaining normal left ventricular function (Fig. 43.2B). Indeed if they are removed during valve replacement, then this results in left ventricular dysfunction (22).
Mitral Valve Pathology: An Integrated Morphologic and Hemodynamic Approach
Although it is tempting to divide pathology into regurgitant and stenotic lesions, this is artificial, since congenital mitral valve abnormalites can result in both. We will therefore describe the types of pathology and their echocardiographic appearances together.
Mitral Valve Dysplasia and Hypoplasia
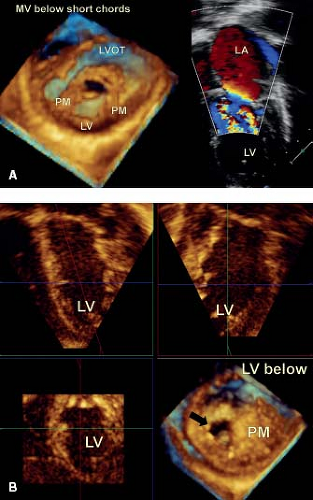
In mitral valve dysplasia, the leaflets are thickened, the interchordal spaces obliterated and the papillary muscles deformed (7,23,24). The valve often shows global hypoplasia and is the most common lesion associated with isolated congenital mitral stenosis, though this may occur in conjunction with regurgitation (Videos 43.1 to 43.4). The free edges of the dysplastic leaflets are thickened and rolled. Therefore, when viewed as a functional unit, the thickened leaflets and obliterated interchordal spaces result in tethering and deficient zones of coaptation. These features are readily appreciated by three-dimensional echocardiography, both from a left atrial and left ventricular view (Fig. 43.3). As well, additional images can be obtained by cropping the heart from above, which demonstrate the mitral valve and its support apparatus. This is of particular value for imaging the chordal apparatus, as they are imaged in the axial plane which provides optimal resolution. The left ventricular view is of particular importance for imaging the commissures, because they are imaged more reliably from below that from the left atrial view. This is due to fact that the normal mitral leaflets billow toward the left atrium, just as a parachute does when seen from the sky.
With the addition of color Doppler, sites of mitral regurgitation can be identified and related to valve pathology (see Fig. 43.3A). It is possible to image the vena contracta, which represents the en face view of the regurgitant jet. The area of this jet correlates well with absolute volume regurgitation, providing a semiquantitative assessment of the degree of regurgitation (Fig. 43.4A). As well, it provides an accurate road map to the location of the regurgitation, providing vital information for the surgeon when planning repair. With the addition of pulsed or continuous wave Doppler the hemodynamic mean gradient can be determined. However, this is directly related to cardiac output, with an underestimation in states of low output. Unfortunately, in the majority of children, it is not possible to calculate a pressure half-time which can provide an absolute valve area in adults.
Dysplasia of the Posterior or Mural Leaflet
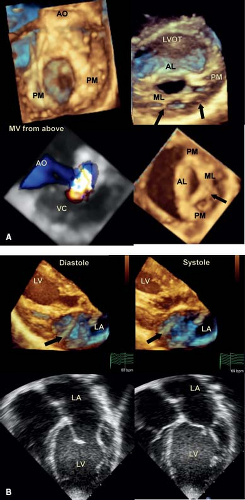
Occasionally, a young child is seen who has significant mitral valve regurgitation secondary to dysplasia of the posterior or mural leaflet (Videos 43.5 and 43.6) (25). The leaflet is invariably tethered by
shortened chordae, preventing normal coaptation with the anterior leaflet (Fig. 43.4). The regurgitant jet extends along the total length of the valve orifice and may respond to surgical repair by leaflet extension. Three-dimensional echocardiography provides optimal imaging of this entity, as well as an accurate assessment of the severity of regurgitation. Although the mural leaflet is tethered, the aortic leaflet is usually thickened (Fig. 43.4).
shortened chordae, preventing normal coaptation with the anterior leaflet (Fig. 43.4). The regurgitant jet extends along the total length of the valve orifice and may respond to surgical repair by leaflet extension. Three-dimensional echocardiography provides optimal imaging of this entity, as well as an accurate assessment of the severity of regurgitation. Although the mural leaflet is tethered, the aortic leaflet is usually thickened (Fig. 43.4).
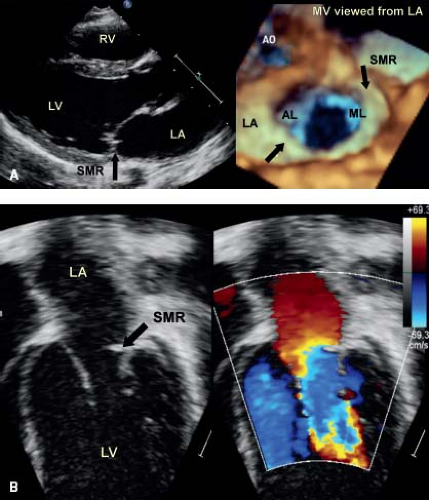
Supravalve Mitral Stenosis
Although this is often considered as a separate entity (Fig. 43.5), it typically coexists with associated abnormalities of the leaflets and subvalve apparatus (26,27) (Fig. 43.6; Videos 43.7 to 43.10). Therefore, in the majority of cases, surgical intervention does not cure the problem, but simply provides some relief of the associated stenosis.
The membrane starts at the annulus of the mitral valve, thus differentiating it from cor triatriatum which sits above the left atrial appendage. It may or may not be circumferential and in some cases extends into the orifice of the mitral valve, providing the appearance of subvalve mitral stenosis. Physiologically, it usually results in mitral stenosis, the hemodynamic consequence which can be determined by a Doppler assessment of the mitral valve in conjunction with an assessment of pulmonary artery pressure from the tricuspid regurgitation and/or pulmonary insufficiency jets. This entity therefore has a physiologic impact on the mitral annulus, as well as the leaflets and subvalve apparatus.
The membrane starts at the annulus of the mitral valve, thus differentiating it from cor triatriatum which sits above the left atrial appendage. It may or may not be circumferential and in some cases extends into the orifice of the mitral valve, providing the appearance of subvalve mitral stenosis. Physiologically, it usually results in mitral stenosis, the hemodynamic consequence which can be determined by a Doppler assessment of the mitral valve in conjunction with an assessment of pulmonary artery pressure from the tricuspid regurgitation and/or pulmonary insufficiency jets. This entity therefore has a physiologic impact on the mitral annulus, as well as the leaflets and subvalve apparatus.
Mitral Arcade
This is a rare entity, however one that has a significant impact on outcome (Videos 43.11 and 43.12). The morphologic and echocardiographic features are of muscularization of the chordal apparatus, such that it is difficult to differentiate between the leaflets, chordae, and their supporting papillary muscles (Fig. 43.7). The functional result is often regurgitation which is due to a tethered valve with deficient zones of leaflet coaptation. This lesion usually presents early on in life and invariably results in a poor outcome (28,29).
Cleft Mitral Valve
This involves the anterior or aortic leaflet of the mitral valve and varies in degree with some hearts having a complete cleft, whereas in others it involves only the tip of the leaflet (Videos 43.13 to 43.17). The cleft points toward the left ventricular outflow tract, which differentiates it from that seen in an atrioventricular septal defect (30,31,32,33,34,35) (Fig. 43.8). The supporting papillary muscles are in the normal location (36), which differs from an atrioventricular septal defect where the posterior muscle is rotated laterally. Mitral valve
regurgitation is usually seen when the edges of the cleft are unsupported by chordae. In other cases, the edges of the cleft are supported by chordae without evidence of associated mitral valve regurgitation (Fig. 43.9). The chordae invariably insert into the crest of the interventricular septum, while in other cases they may straddle an anterior ventricular septal defect. The degree of mitral valve regurgitation is usually dictated by the extent of the cleft, with greater regurgitation in those where the cleft extends along the whole length of the anterior leaflet. While two-dimensional echocardiography is helpful in the diagnosis, it does not permit a complete evaluation of the extent of the cleft: three-dimensional echocardiography does. Imaging the mitral valve from the left atrial or left ventricular aspect provides a complete assessment of the extent of the cleft, the supporting apparatus and the commissures. The site and degree of regurgitation can be determined by an en face view of the valve using color Doppler.
regurgitation is usually seen when the edges of the cleft are unsupported by chordae. In other cases, the edges of the cleft are supported by chordae without evidence of associated mitral valve regurgitation (Fig. 43.9). The chordae invariably insert into the crest of the interventricular septum, while in other cases they may straddle an anterior ventricular septal defect. The degree of mitral valve regurgitation is usually dictated by the extent of the cleft, with greater regurgitation in those where the cleft extends along the whole length of the anterior leaflet. While two-dimensional echocardiography is helpful in the diagnosis, it does not permit a complete evaluation of the extent of the cleft: three-dimensional echocardiography does. Imaging the mitral valve from the left atrial or left ventricular aspect provides a complete assessment of the extent of the cleft, the supporting apparatus and the commissures. The site and degree of regurgitation can be determined by an en face view of the valve using color Doppler.
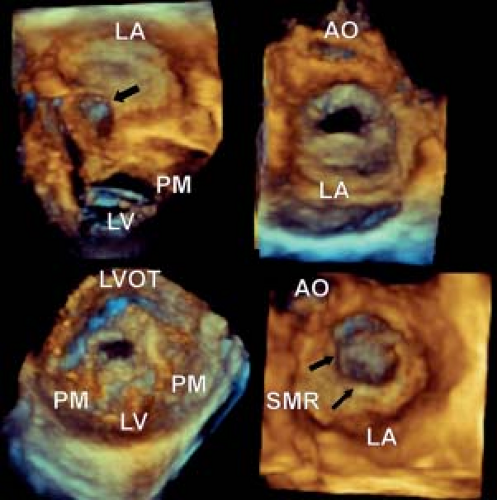
Parachute Mitral Valve
Although cases exist with a solitary papillary muscle, the initial description by Shone (26) included hearts with a dominant papillary muscle which supported most of the chordal apparatus and a smaller secondary rudimentary muscle. The presence of a parachute mitral valve does not infer stenosis or regurgitation, as medium term data would suggest that many require no intervention (37). In general it is the associated leaflet dysplasia and chordal tethering which result in valve failure. This entity is readily recognized by two-dimensional echocardiography, however its three-dimensional counterpart permits a more detailed assessment of the valve leaflets and chordal apparatus.
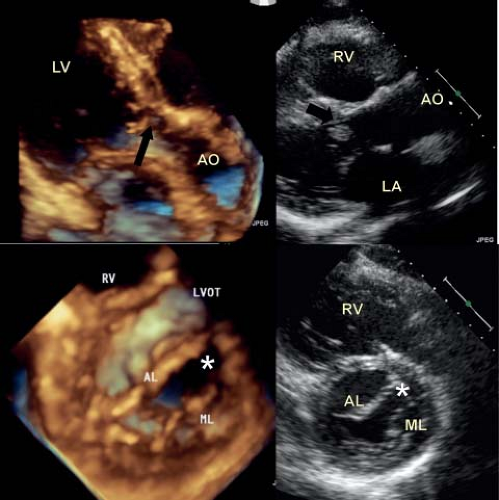
Double Orifice Mitral Valve
This entity is seen more frequently in hearts with an atrioventricular septal defect, however is occasionally encountered in an otherwise normal heart (Videos 43.18 and 43.19) (38,39). In this setting it is more common for each orifice to be supported by chordal apparatus and papillary muscle (Fig. 43.10A). This may be discovered as an incidental finding during an echocardiogram for another reason, while in other cases one of the functional orifices is regurgitant. These valves are rarely stenotic and in most cases never require any intervention. In general this entity is readily recognized by both two- and three-dimensional echocardiography. In some instances one of the orifices is imperforate, and the supporting tension apparatus can be appreciated from the left ventricular aspect (Fig. 43.10B).
Ebstein’s-Like Anomaly of the Mitral Valve
This is a rare entity that involves displacement of the posterior or mural leaflet without thinning of the atrialized portion of the left ventricle. It presents in early infancy and is associated with high
mortality, particularly when there is associated aortic arch hypoplasia or coarctation (40).
mortality, particularly when there is associated aortic arch hypoplasia or coarctation (40).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree