50 An Approach to Children with Suspected Congenital Heart Disease
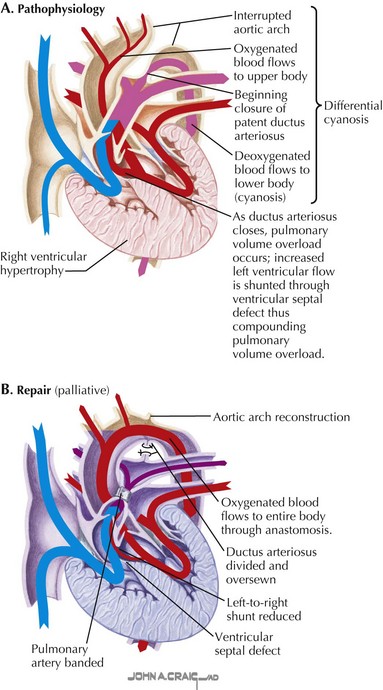
Congenital heart defects can be classified into those that result in cyanosis and those that do not. Acyanotic defects include those with a left-to-right shunt and increased pulmonary blood flow and obstructive defects without associated shunting. Left-to-right shunts occur at various anatomic levels: atrial (e.g., atrial septal defect), ventricular (e.g., ventricular septal defect—part of the complex defect depicted in Fig. 50-1), or arterial (e.g., patent arterial duct). Obstructive lesions without any associated shunts include pulmonary stenosis, aortic stenosis, and coarctation of the aorta.
Cyanotic defects are generally characterized by a right-to-left shunt and may be classified into two broad categories. In the first group, with intracardiac defects and obstruction to pulmonary flow, cyanosis results from decreased pulmonary blood flow and the intracardiac mixing of oxygenated and desaturated blood. In the second group, cyanosis results from the admixture of pulmonary and systemic venous returns despite normal or increased pulmonary blood flow. In most cardiac malformations classified in this group, a single chamber receives the total systemic and pulmonary venous returns. The mixing of oxygenated and desaturated blood can occur at any level: venous (e.g., total anomalous pulmonary venous connection), atrial (e.g., single atrium), ventricular (e.g., single ventricle), and great vessel (e.g., persistent truncus arteriosus). In all these circumstances, near-uniform mixing of the venous returns usually occurs. Complete transposition of the great arteries (Fig. 50-2) can be included in this group, although only partial admixture of the two venous returns occurs, leading to severe hypoxemia.
Clinical Indications for Medical or Surgical Intervention
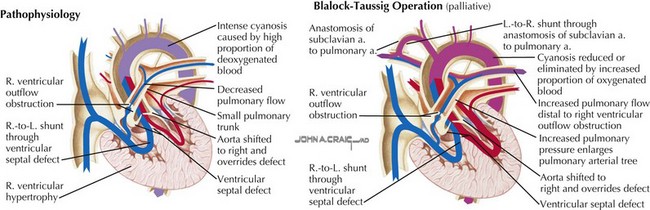
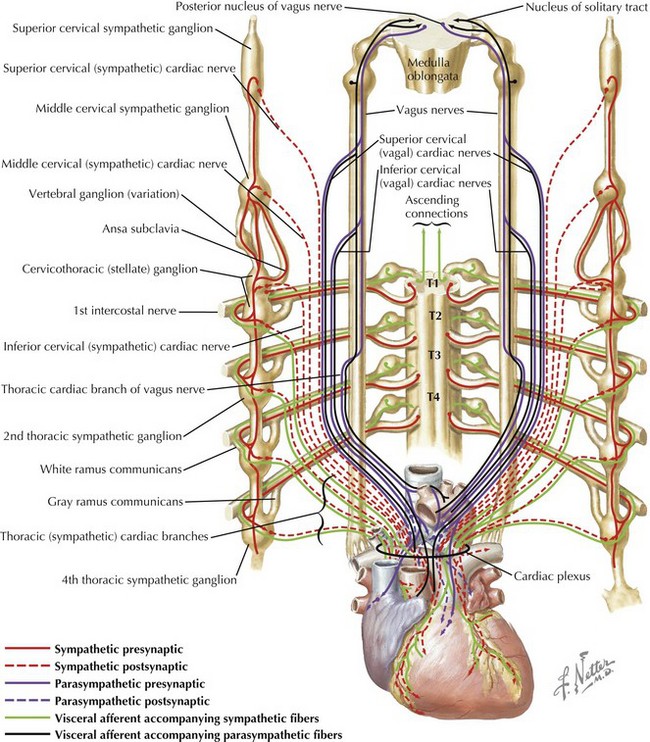
The interdisciplinary approach that is needed clinically to optimally care for children with congenital heart disease includes accurate assessment of anatomic defects and their physiologic consequences and effective communication of these findings. Management of congenital heart disease revolves around manipulating abnormal pulmonary and systemic blood flows. The consequences of altered blood flow induced by congenital heart disease and the effects of therapeutic interventions invariably influence the pulmonary circulation by increasing pulmonary blood flow (e.g., left-to-right shunting through intracardiac septal defects), decreasing pulmonary blood flow (e.g., right-sided obstructive heart lesions, such as tetralogy of Fallot) (Fig. 50-3), altering the pathway of pulmonary blood flow (e.g., Fontan-Kreutzer repair), or altering the hemodynamics to which pulmonary blood flow (e.g., pulmonary hypertension) is subjected. The clinician must be able to manage such conditions in which there is increased pulmonary blood flow or a paucity of pulmonary blood flow and the associated repercussions as to how they relate to the systemic circulation. Obtaining the optimal balance between these two circulations, which are frequently not in series in the case of congenital heart disease, requires the ability to monitor pulmonary hemodynamics and assess pulmonary vascular impairment successfully. Critically important to an understanding of the physiologic consequences of these defects are the maturational differences that occur in cardiopulmonary function during a child’s development. For example, cardiac function is subject to maturational changes occurring at the cellular level in a variety of processes, including those in the neurocardiac functional unit: changes in neurotransmitter content, the receptor system, innervation, the effector-transducer systems, and the cellular components that are affected by autonomic stimulation (Fig. 50-4). These changes affect the strategies that can be utilized to successfully manage the sequelae of congenital heart disease.
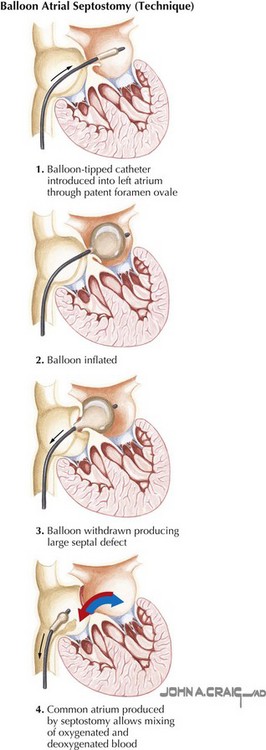
Defining the pathophysiology of pulmonary vascular disease remains an important area of research. The primary approaches used today involve therapeutic interventions to eliminate the risk factors for pulmonary vascular disease in all children identified at high risk. Three principal risk factors must be characterized in the assessment of congenital heart disease: increased pulmonary blood flow from left-to-right intracardiac or extracardiac shunting (e.g., septal defect, patent arterial duct, arteriovenous fistula, transposition of the great arteries) (Fig. 50-5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree