We read the recent meta-analysis by Jang et al of 3 randomized trials and 9 observational studies (with 5,079 patients) comparing percutaneous coronary intervention (PCI) with drug-eluting stents (DES) versus coronary artery bypass grafting (CABG) for unprotected left main coronary artery (ULMCA) disease. At 1-year follow-up, there were trends toward lower risk for death (odds ratio [OR] 0.68, 95% confidence interval [CI] 0.45 to 1.02, p = 0.06) and the composite end point of death, myocardial infarction, or stroke (OR 0.70, 95% CI 0.49 to 1.00, p = 0.05) in the DES group compared to the CABG group. However, target vessel revascularization (TVR) was significantly higher in the DES group compared to the CABG group (OR 3.52, 95% CI 2.72 to 4.56, p <0.00001). The investigators concluded that PCI with DES was associated with favorable outcomes for mortality and the composite end point of death, myocardial infarction, or stroke and with a higher risk for TVR compared to CABG in patients with ULMCA disease. However, the investigators abstracted unadjusted dichotomous data (events/total) in unmatched groups from observational studies except for the Revascularization for Unprotected Left Main Coronary Artery Stenosis: Comparison of Percutaneous Coronary Angioplasty Versus Surgical Revascularization (MAIN-COMPARE) study which consisted propensity score-matched groups. Unadjusted risk estimates in observational studies must be highly biased. Thus, extracting not “unadjusted” but “adjusted” risk estimates from observational studies, we performed an updated meta-analysis of PCI with DES versus CABG for the prevention of late death and major adverse cardiac and cerebrovascular events (MACCEs; a composite of death, myocardial infarction, or stroke) in ULMCA disease. All analyses were conducted using Review Manager version 5.1 (Nordic Cochrane Centre, Copenhagen, Denmark) and Comprehensive Meta-Analysis version 2 (Biostat, Englewood, New Jersey).
Our comprehensive search, using the same strategy as Jang et al, through October 2012 identified 3 randomized trials and 19 observational studies reporting adjusted risk estimates for late (≥12-month) death and MACCEs. We selected risk estimates at the longest follow-up point in each individual study. In total, our meta-analysis included data on 9,217 patients with ULMCA disease assigned to PCI with DES or CABG. We abstracted unadjusted hazard ratios (HRs) and risk ratios (instead of HRs) from randomized trials and adjusted HRs and ORs (instead of HRs) from observational studies. Adjustment methods in observational studies included propensity score analysis and/or logistic or Cox proportional-hazard regression. Pooled analysis demonstrated no statistically significant difference in the risk for death (fixed-effects HR 1.01, 95% CI 0.86 to 1.18, p for effect = 0.88, p for heterogeneity = 0.32, p for subgroup [randomized trial and observational study subgroups] differences = 0.23; Figure 1 ) and MACCEs (fixed-effects HR 0.98, 95% CI 0.86 to 1.13, p for effect = 0.26, p for heterogeneity = 0.30, p for subgroup differences = 0.31; Figure 2 ) between patients assigned to PCI with DES and those assigned to CABG. There was minimal between-study heterogeneity and accordingly little difference in the pooled results of random-effects modeling. Sensitivity analyses were performed to assess the contribution of each study to the pooled estimate by excluding individual studies 1 at a time and recalculating the pooled HR estimates for the remaining studies. In general, the exclusion of any single study from the analysis did not substantively alter the overall results of our analysis. There was evidence of significant publication bias (2-tailed p values for death and MACCEs = 0.00861 and 0.01734, respectively, by Begg adjusted rank-correlation test with continuity correction). For specific evaluation of the presence and extent of publication bias, we used the trim-and-fill method according to Duval and Tweedie, which imputes missing studies in the funnel plot on the basis of symmetry assumptions. Trim-and-fill analysis demonstrated that 5 and 4 missing studies for death and MACCEs were needed to achieve a symmetric funnel plot, respectively. The pooled analysis incorporating the hypothetical studies showed no statistically significant difference in the risk for late death (fixed-effects HR 1.09, 95% CI 0.93 to 1.26) and MACCEs (fixed-effects HR 1.05, 95% CI 0.92 to 1.20).

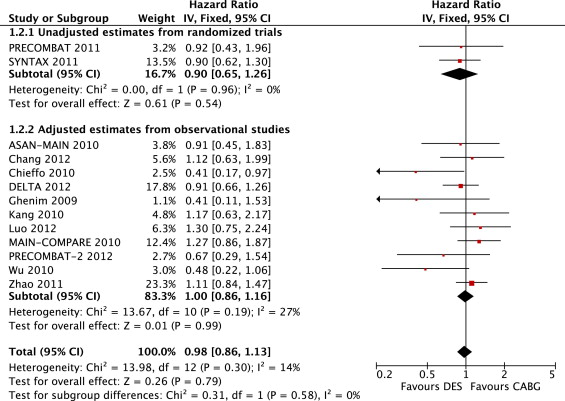
Despite the results of the meta-analysis by Jang et al, the present analysis suggests that PCI with DES may reduce neither late death nor MACCEs (not including TVR) compared to CABG in patients with ULMCA disease, which was robust in sensitivity analyses and even after adjustment for publication bias. Another recent meta-analysis of 12 adjusted observational studies showed at ≤2-year follow-up that PCI with DES and CABG for ULMCA disease were not different in risk for mortality (risk ratio 0.83, 95% CI 0.53 to 1.28, p = 0.39) and MACCEs including TVR (risk ratio 1.22, 95% CI 0.86 to 1.73), which may strengthen our results. Compared to randomized trials, potential biases are likely to be greater for nonrandomized observational studies. Randomization with adequate allocation sequence concealment reduces the possibility of systematic selection bias so that differences in characteristics between groups can be attributed to chance, whereas allocation to groups depends on other factors (often unknown) in observational studies. Confounding occurs when selection bias gives rise to imbalances between intervention and control groups on prognostic factors; that is, the distributions of the factors differ between groups, and the factors are associated with outcomes. Unlike for randomized trials, it would usually be appropriate to analyze “adjusted,” rather than “unadjusted,” risk estimates (i.e., analyses that attempt to control for confounding). Thus, researchers should abstract not “unadjusted” but “adjusted” risk estimates from observational studies and then combine them in their meta-analyses.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


