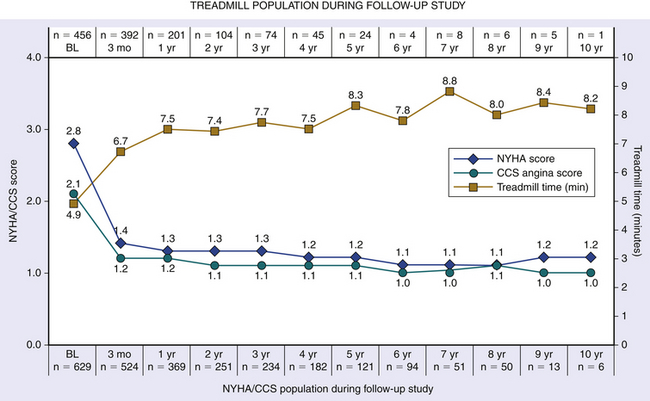
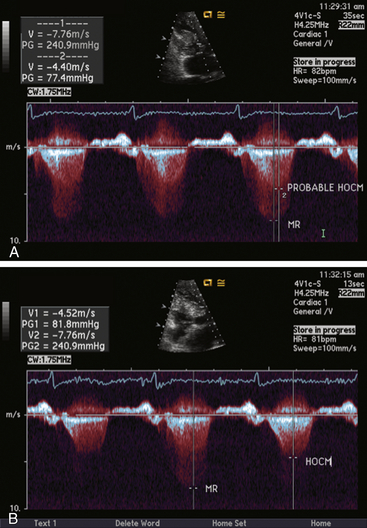
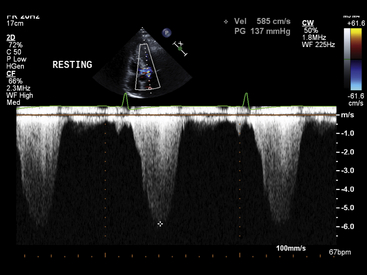
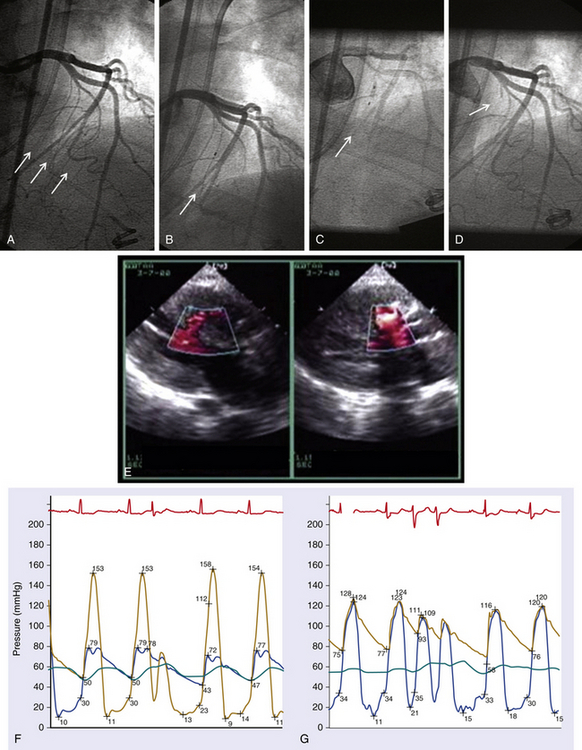
Chapter 14 • In appropriate patients with hypertrophic cardiomyopathy (HCM), alcohol septal ablation using contrast echocardiographic guidance causes targeted necrosis, thinning of the basal interventricular septum, and widening of the left ventricular outflow tract (LVOT) and relieves outflow tract obstruction. • Alcohol septal ablation is indicated as an alternative option for septal reduction therapy for patients with HCM who have significant symptoms refractory to medical therapy and attributable to severe LVOT obstruction that results from asymmetric septal hypertrophy (ASH) and systolic anterior motion (SAM) of the mitral valve. • Patients at higher risk for surgical myectomy, including older patients and those with comorbidities, and symptomatic patients who have not obtained a satisfactory result after septal myectomy, may be excellent candidates for alcohol septal ablation. • Alcohol septal ablation may be complicated by high-degree atrioventricular (AV) block, requiring permanent pacemaker (PPM) implantation in approximately 10% of patients and ventricular tachyarrhythmias in up to 3% of patients and should be performed in experienced centers. HCM is the most common cardiovascular genetic disorder, characterized by unexplained cardiac hypertrophy involving a nondilated left ventricle in the absence of other identifiable cardiac or systemic causes. The location and extent of myocardial hypertrophy can vary among patients with HCM, but the most common phenotype involves ASH of the basal interventricular septum that may be accompanied by dynamic obstruction of the LVOT. Although there is wide anatomic and physiologic heterogeneity among patients with HCM and variability in the clinical manifestations, the presence of dynamic LVOT obstruction has been related to symptomatic status and a higher risk of death.1,2 In patients with appropriate anatomic features and significant dynamic LVOT obstruction associated with severe and limiting symptoms refractory to medical therapy, treatment with septal reduction therapy may be considered to improve quality of life. Septal reduction for patients with HCM can be accomplished by one of two procedures: (1) surgical septal myectomy or (2) percutaneous transcatheter alcohol septal ablation. Whereas septal myectomy has been recommended as a preferred method of septal reduction for many patients (especially younger patients), for patients at higher surgical risk or who prefer to avoid surgery, alcohol septal ablation represents an effective treatment option that can provide similarly successful symptom relief and improved quality of life.3 Alcohol septal ablation was first reported by Ulrich Sigwart in 1995.4 Sigwart had made the seminal observation that the degree of LVOT obstruction in patients with HCM responded favorably to ischemia caused by temporary balloon occlusion of a major septal artery, but that it returned to baseline after balloon deflation. In this first report, in three cases he was able to successfully and durably reduce the LVOT obstruction by inducing targeted necrosis of the basal interventricular septum via selective injection of alcohol into a septal artery and achieve sustained relief of symptoms that had previously been refractory to medical management. The conceptual approach is illustrated in Figure 14–1. In subsequent reports of early experience with alcohol septal ablation in relatively small case series, successful LVOT gradient reduction and improvement in symptoms was demonstrated, although it was associated with a relatively high incidence (30% to 40%) of high-degree AV block, requiring PPM insertion.5–8 After the initial series of patients, a technical advance was recognized, whereby intraprocedural contrast echocardiographic mapping of the perfusion territory of the candidate septal artery was incorporated into the procedure,9 allowing more selective and precise localization of the target territory appropriate for ablation. This was associated with reduction in the rate of complications, including AV block. Since its introduction, alcohol septal ablation has been used successfully worldwide to treat a large number of severely symptomatic patients with HCM and LVOT obstruction, including patients who have undergone a previously unsuccessful surgical myectomy.10 Figure 14–1 Transcatheter alcohol septal ablation. With respect to procedural success and outcomes, several studies have confirmed that significant reduction of the LVOT gradient and improvement of symptoms are accomplished by alcohol septal ablation in 90% or more of patients.11,12 Recent meta-analysis and reports with longer-term follow-up periods have supported immediate, short-term and sustained long-term gradient reduction and symptomatic improvement after alcohol septal ablation.13–17 Despite the absence of randomized trial data, comparisons of contemporary case series suggest that the symptomatic improvements after alcohol septal ablation appear similar to those reported after myectomy, although alcohol septal ablation is associated with a somewhat higher posttreatment LVOT gradient and higher risk of PPM implantation. A systematic review18 of 42 published studies analyzing results from 2959 patients undergoing alcohol septal ablation from 1996 to 2005, with an average follow-up time of about 1 year, reported a sustained reduction of the resting LVOT gradient from 65 mmHg to 16 mmHg and of the provoked LVOT gradient from 125 mmHg to 32 mmHg, associated with a significant improvement in exercise capacity and New York Heart Association (NYHA) functional class (from a mean of 2.9 to 1.2). Long-term follow-up data from a substantial number of patients undergoing alcohol septal ablation have been reported from several centers since 2008. Welge et al19 reported that for 347 patients who underwent alcohol septal ablation at one center in Germany, at a follow-up period of over nearly 5 years 89% were symptomatically improved with NYHA class I or II symptoms, 74% were free of LVOT obstruction at rest, and 60% did not exhibit any provocable LVOT obstruction. Fernandes and colleagues14 reported long-term outcomes of alcohol septal ablation performed in 629 patients at two centers in the United States from 1996 to 2007. The mean follow-up period was 4.6 ± 2.5 years and ranged from 3 months to 10.2 years. In that series, there appeared to be a progressive decline in the LVOT gradient over the long-term follow-up period, with the mean resting gradient at baseline of 77 ± 31 mmHg decreasing to 26 ± 27 mmHg at 3 months, 20 ± 24 mmHg at 1 year, and less than 10 mmHg in those tested after 5 years (p < 0.001). During follow-up study, NYHA functional class decreased from the baseline of 2.8 ± 0.6 to 1.2 ± 0.5 (p < 0.001); Canadian Cardiovascular Society (CCS) angina class decreased from 2.1 ± 0.9 to 1.0 ± 0 (p < 0.001); and exercise time increased from 4.8 ± 3.3 to 8.2 ± 1.0 minutes (p < 0.001) (Figure 14–2). The survival estimates were favorable at 1, 5, and 8 years at 97%, 92%, and 89%, respectively. The rate of new pacemakers required for high-degree heart block was 9.7%. Within this cohort, 14% underwent repeat alcohol septal ablation and 4% underwent myectomy for unsatisfactory initial results. Sorajja et al20 reported outcomes from 138 patients who underwent alcohol septal ablation at the Mayo Clinic from 1999 to 2006. In that series, the 4-year survival rate free of death and severe NYHA class III/IV symptoms after alcohol septal ablation was 76.4%, and 71 patients (51%) became asymptomatic. They noted, however, from a nonrandomized comparison, that the rate of procedural complications appeared higher than in age- and gender-matched patients who had undergone septal myectomy at the Mayo Clinic, especially among younger patients. Jensen and colleagues21 recently reported long-term outcomes of alcohol septal ablation among 279 patients with HCM, many of whom had significant comorbidities, performed from 1999 to 2010 in four Scandinavian centers. In their experience, the median LVOT gradient at rest was reduced by alcohol septal ablation from 58 to 12 mmHg at 1-year (p < 0.001), and the gradient provoked by Valsalva maneuver was reduced from 93 to 21 mmHg (p < 0.001). The proportion of patients with NYHA class III/IV symptoms was reduced from 94% to 21% (p < 0.001), and the proportion of patients with syncope was reduced from 18% to 2% (p < 0.001). In-hospital mortality was low at 0.3%. The 1-, 5- and 10-year survival rates were 97%, 87%, and 67%, respectively (p < 0.06 vs. an age- and sex-matched background population). Figure 14–2 Long-term follow-up study after alcohol septal ablation among 629 patients with hypertrophic cardiomyopathy including functional score (NYHA heart failure class and CCS angina class) and treadmill exercise time showing marked early improvement of heart failure symptoms, angina, and treadmill exercise time at 3 months that persisted during the follow-up period. (BL, Baseline; CCS, Canadian Cardiovascular Society; NYHA, New York Heart Association.) (Reproduced with permission from Fernandes, VL, Nielsen, C, Nagueh, SF, et al: Follow-up of alcohol septal ablation for symptomatic hypertrophic obstructive cardiomyopathy the Baylor and Medical University of South Carolina experience, 1996 to 2007. JACC Cardiovasc Interv 1:561-70, 2008.) A report of a large-scale North American registry13 has provided additional important information from a multicenter experience involving 874 patients undergoing alcohol septal ablation at nine centers from 2000 to 2010. Before the procedure nearly 80% of patients had NYHA class III or IV heart failure symptoms; 43% of patients had CCS class III or IV angina; and 29% reported syncope. After the procedure, there was significant symptomatic improvement, with fewer than 5% of patients having NYHA class III or IV heart failure, less than 1% of patients having CCS class III angina (none had class IV), and only 2.9% of patients with syncope. Among the patients in the registry, survival estimates at 1, 5, and 9 years were 97%, 86%, and 74%, respectively. The authors noted that the overall survival rate at 1 year after alcohol septal ablation (97% vs. 98%) was similar to that seen in a disease-free general population, and survival at 1, 5, and 9 to 10 years appeared better after alcohol septal ablation compared with patients reported in other series, who had HCM and did not undergo septal reduction therapy.22 Despite the high success rate of alcohol septal ablation, a small proportion (<10%) of patients may not achieve adequate hemodynamic or clinical response. Among several potential causes, insufficient necrosis and thinning of the basal septal hypertrophy may be the most common etiology. Patients who undergo alcohol septal ablation and are found at follow-up study to have significant residual LVOT gradients, and unrelieved or recurrent symptoms may benefit from a second septal reduction procedure. Among patients in the reported series, approximately 6% to 14% underwent repeat alcohol septal ablation, with reportedly favorable hemodynamic and symptomatic outcomes,13,18,19,21,23,24 and 2% to 3% underwent surgical myectomy, also with favorable outcomes but a possibly higher risk of postoperative pacemaker requirement caused by AV block, compared with patients undergoing myectomy without a prior history of septal ablation.25 It should be noted, however, that in the North American registry, multivariate analysis suggested that repeat alcohol septal ablation procedures may be associated with higher long-term mortality risk.13 The diagnosis of HCM generally relies on high-quality echocardiographic detection of the characteristic asymmetric left ventricular (LV) hypertrophy. HCM is diagnosed when maximal LV wall thickness is 15 mm or greater in the absence of other identifiable causes, whereas wall thickness of 13 to 14 mm may be considered borderline.3 The most common phenotype of HCM relevant for consideration of septal reduction includes disproportionate hypertrophy of the basal interventricular septum (ASH), such that the ratio of the septal to posterior wall thickness is greater than 1:3 (Figure 14–3). This basal septal hypertrophy typically narrows the LVOT and may be accompanied by SAM of the anterior mitral valve leaflet. During ventricular systole, as flow accelerates across the basal hypertrophied septum, drag forces and a Venturi effect are created where the anterior leaflet of the mitral valve is directed towards that segment of the septum, resulting in mitral–septal contact. In a substantial proportion of patients, ASH and SAM contribute to significant dynamic LVOT obstruction, although the degree of obstruction to LV outflow varies across a wide spectrum. It has been estimated that one quarter to one third of patients show LVOT obstruction at rest, although when adequate provocative testing is performed, the majority of patients with HCM (≥70%) are found to have significant resting or provocable obstruction.26 Figure 14–3 Parasternal long-axis view indicates an asymmetrically thickened left ventricular myocardium. The evaluation of patients with HCM for consideration of eligibility for septal reduction therapy includes two-dimensional echocardiography, employing a protocol that fully investigates these relevant anatomic and physiologic features. This includes a focused assessment of LV morphology, septal thickness, and dynamic LVOT obstruction at rest and with stress. The examination may be best accomplished with a specialized stress echocardiogram protocol, providing an objective assessment of exercise tolerance and an assessment of LVOT velocity at peak physiologic stress. Dynamic LVOT obstruction is most commonly quantified using Doppler echocardiographic interrogation of the LVOT, with care to avoid contaminating the spectral display with accelerated velocity from mitral regurgitation, which can confound accurate estimation of the LVOT gradient (Figure 14–4). With LVOT obstruction in HCM the spectral Doppler display shows a characteristic accelerated velocity with a late-peaking dagger-shaped appearance (Figure 14–5). Because intraventricular obstruction can be present from other causes such as fixed subaortic stenosis or midventricular obstruction in the setting of cavity obliteration, care is warranted in identifying the site and etiology of obstruction. The systolic pressure gradient resulting from LVOT obstruction is proportional to the peak instantaneous LVOT velocity and can be reliably estimated using the modified Bernoulli formula. Because LVOT obstruction is dynamic and may not be present at rest in many patients, provocative maneuvers are necessary for a comprehensive evaluation of LVOT obstruction in patients with symptoms suspected because of HCM. Doppler imaging during Valsalva maneuver and amyl nitrite inhalation can elicit a provoked gradient, although upright exercise with immediate assessment of the peak LVOT velocity likely represents the most physiologically sound method to estimate the relevant peak provocable LVOT gradient. Combining a formal exercise stress protocol with immediate postexercise Doppler echocardiography is the authors’ preferred method for determining the presence and quantifying the degree of physiologically relevant provocable LVOT obstruction, and it is especially useful in evaluating patients with symptoms who do not have resting outflow obstruction or whose degree of resting LVOT obstruction is only mild to moderate (i.e., <50 mmHg).26 For patients who cannot exercise, graded dobutamine infusion has been used as a method to estimate peak provocable gradients by a readily reproducible protocol, but because of concerns regarding its potential lack of specificity and physiologic relevance, its use in selection of patients for septal reduction has been controversial. Figure 14–4 A, Continuous wave pulse Doppler through the left ventricular outflow tract (LVOT) can often be difficult, because the mitral regurgitant velocities can interfere with measurement. B, Moving the site of Doppler analysis at the time of recording can help differentiate mitral regurgitant velocities from LVOT velocity. Resting LVOT gradient is approximately 80 mmHg. Figure 14–5 Continuous wave Doppler through the LVOT. The accelerated velocity has a characteristic late-peaking dagger-shaped appearance. The Doppler signal demonstrates a resting gradient of 137 mmHg. Transesophageal echocardiography (TEE) also has an important role in the evaluation of patients with HCM to assess coincident mitral regurgitation and mitral valve morphology and to exclude any form of fixed LVOT obstruction that may be unexpectedly present in a small number of patients carrying a diagnosis of HCM referred for septal reduction.27 TEE can help define mitral valve, chordal and papillary muscle anatomy, and the degree and etiology of mitral regurgitation. For patients in whom severe mitral regurgitation is observed, some features of the visualized regurgitant jet (e.g., eccentricity and posterior direction, responsiveness to pharmacologic maneuvers) and mitral valve morphology can be helpful in determining whether the mitral regurgitation is likely secondary to LVOT obstruction and SAM (when it is likely to improve with relief of LVOT obstruction) or whether it is the result of independent mitral valve pathology, when indications favoring surgical valvular intervention may also be present.28 With respect to any suspicion of fixed LVOT obstruction, also termed discrete subaortic stenosis, the subaortic ridge or membrane may not be visible on two-dimensional echocardiographic images, whereas coincident septal hypertrophy may resemble traditional HCM, leading to mistaken diagnosis and treatment.27 Clues to the presence of a subaortic membrane or some form of fixed LVOT obstruction on two-dimensional imaging include absence of SAM or an accelerated LVOT velocity that is early peaking despite absence of morphologic features of aortic stenosis, often associated with mild to moderate aortic insufficiency. When suspected, additional imaging by TEE or cardiac magnetic resonance imaging is strongly recommended to exclude fixed LVOT obstruction before consideration of alcohol septal ablation. Cardiac catheterization is also routinely performed in the evaluation of candidacy for septal reduction therapy, providing an angiographic assessment of coincident coronary artery disease and septal artery anatomy and allowing a thorough catheter-based assessment of the dynamic intraventricular obstruction, any of which may affect management strategy. In patients with anginal chest pain, coronary angiography is essential to assess the presence of coincidental coronary artery disease. The catheter-based evaluation of ventricular hemodynamics may also be especially important when the results of rest or provoked gradient assessment by Doppler echocardiography are equivocal or unobtainable. Hemodynamic assessment can be readily performed using small-caliber catheters and two transducers to measure simultaneous intraventricular and aortic or femoral artery pressure. For intraventricular measurements, a pigtail catheter should be avoided because of side holes extending several centimeters along the distal end, potentially crossing the site of obstruction and artifactually lowering the LVOT gradient, and end-hole catheters raise a theoretical concern of catheter entrapment and artifactually increased intraventricular pressure. Catheters with end and side holes limited to the distal 1 to 2 cm (e.g., multipurpose A-2 or Halo pigtail catheter) may be best suited for this evaluation. Simultaneous LV and aortic or femoral artery pressures are measured at rest, after ventricular extrasystoles, during Valsalva maneuver, and after amyl nitrite inhalation to fully profile the resting and provocable LVOT gradients. The Brockenbrough-Braunwald-Morrow sign, a simultaneous increase in the LVOT systolic gradient associated with a diminished aortic pulse pressure after a ventricular extrasystole that is likely the result of postextrasystolic potentiation of LV contractility, is the classic manifestation of the dynamic LV–aortic gradient, and remarkably high gradients can be seen (Figures 14–6 and 14–7). A careful pullback can demonstrate the intraventricular location of the systolic gradient and confirm the absence or measure the amount of any aortic valve–related systolic gradient in the presence of coincident aortic valve stenosis (Figure 14–8). Figure 14–6 A, Alcohol septal ablation procedure: the baseline left coronary angiogram shows 3 candidate septal arteries originating from the proximal LAD (arrows). B, The second septal artery is cannulated with a balloon (arrow), but contrast echo imaging via the inflated balloon shows enhancement of the midseptum remote from the ASH and SAM (E, left). C, The balloon is then repositioned into the first septal artery and inflated (arrow), and repeat contrast echo (with power harmonic) imaging shows enhancement of the basal septum at the site of septal–SAM contact (E, right). D, Then 2.5 cc of alcohol is injected over 10 minutes; after later balloon removal, repeat angiography shows typical proximal occlusion of the ablated first septal (arrow). The lower graphs show the patient’s LVOT hemodynamics with and without ventricular extrasystoles before (F) and immediately after (G) alcohol septal ablation, showing immediate near elimination of the resting and provoked LVOT gradient by alcohol septal ablation.
Alcohol Septal Ablation for Obstructive Hypertrophic Cardiomyopathy
14.1 Key Points
14.2 Background
Alcohol Septal Ablation: Historical Considerations
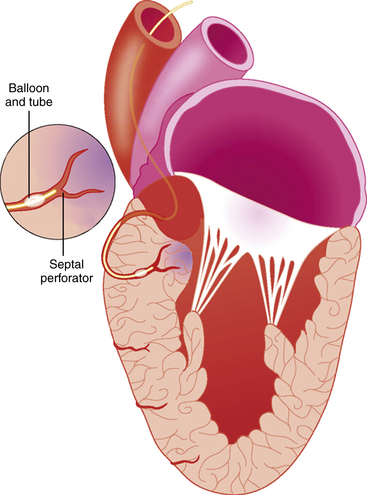
The illustration shows an inflated angioplasty balloon occluding the first septal perforator branch of the left anterior descending artery (LAD) with a targeted area of necrosis in the perfused basal septum caused by injection of alcohol via the balloon catheter.
Outcomes
14.4 Patient Evaluation
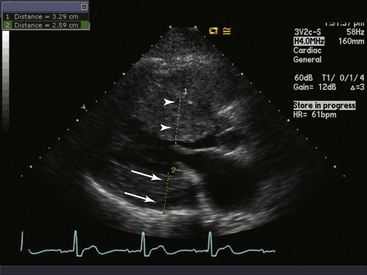
The septal myocardium (arrowheads) is thickened at approximately 3.3 cm, whereas the posterior wall (arrows) is thickened at 2.6 cm. The ratio of the septal to posterior wall is approximately 1:3.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
