14

Adult Respiratory Distress Syndrome
The adult respiratory distress syndrome (ARDS) is a complex clinical, radiologic, and physiologic syndrome1–4 that is characterized by severe respiratory failure in a variety of clinical settings (Table 14–1), and therefore has been referred to by a variety of names (Table 14–2). Although the syndrome was first described in detail by Ashbaugh et al,5 it had been known previously, particularly in battle field conditions.6 Several other reports in the older literature were most likely ARDS interpreted wrongly as rapidly progressive fibrosing alveolitis7,8 or chemical pneumonia.9 The patients described by Ashbaugh et al had acute respiratory distress that occurred after a variety of initial insults and showed clinical, physiologic, and pathologic features secondary to the presence of pulmonary edema despite low or normal pulmonary vascular hydrostatic pressures. Ashbaugh et al also noted some similarities to the respiratory distress syndrome of the newborn, then called hyaline membrane disease, notably the presence of hyaline membranes. During the Vietnam War, the syndrome became very common, as victims received early treatment of their injuries at medical evacuation stations, only to succumb from intractable respiratory failure while in the hospital.
Diagnosing ARDS usually requires the following: (1) presence of appropriate risk factors, (2) presence of bilateral infiltrates on chest x-ray, (3) marked impairment of gas exchange requiring mechanical ventilation, and (4) absence of congestive heart failure. Based on evidence indicating that ARDS is the most severe result of a multiplicity of insults that produce acute injury followed by repair, Murray and colleagues10 have suggested that such a stringent definition is not appropriate, and patients could best be classified by using a lung injury scoring system based on the extent of changes in the chest radiograph, the severity of both hypoxemia and the loss of lung compliance, and the amount of positive end-expiratory pressure (PEEP) needed for adequate ventilation. However, the 1994 consensus statement from the American Thoracic Society and the European Society of Intensive Care Medicine has individually listed the requirements for a diagnosis of acute lung injury or of ARDS.11 Although both diagnoses require (1) acute onset, (2) bilateral infiltrates on radiograph, (3) a capillary wedge pressure of < 19 mm torr, and (4) no clinical evidence of left atrial hypertension, the ratio of partial pressure of arterial O2 to FiO2 is <300 torr in acute lung injury, and <200 torr in ARDS.11
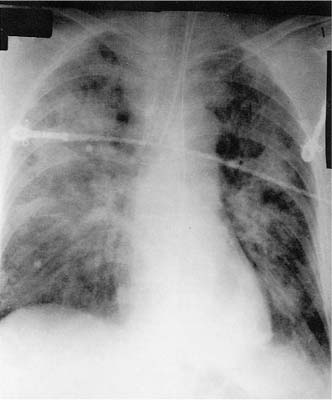
Clinical events, common to all patients, include severe dyspnea, tachypnea, cyanosis refractory to oxygen therapy, loss of lung compliance, and a diffuse alveolar infiltrate on chest radiograph. The initial features of respiratory distress occur between 1 hour and 4 days after the precipitating event, and consist of shortness of breath that rapidly worsens until the patient requires ventilatory support. The arterial PO2 is decreased, and often requires a high concentration of oxygen to maintain adequate levels. The chest radiograph is generally unremarkable in the early (latent) phase12 if the etiology is not a direct pulmonary insult. In the next stage (acute) the radiograph begins to show increasing densities, first primarily interstitial, and then both interstitial and alveolar in nature, progressing toward “total white out” (Fig. 14–1). These radiographic changes may occur after difficulties in oxygenation, or may be altered by the effects of PEEP. The patient becomes increasingly difficult to ventilate, often requiring high ventilatory pressures and increasing levels of PEEP to administer a relatively small tidal volume.
1. Shock ofg any cause 2. Infections a. Sepsis of any type b. Viral pneumonia c. Fungal pneumonia d. Severe bacterial pneumonia e. Pneumocystis pneumonia 3. Trauma and related damage a. Crush, blast injury b. Major bone fractures c. Head trauma d. Lung contusions e. Fat emboli f. Massive blood transfusions g. Disseminated intravascular coagulopathy 4. Aspiration a. Gastric contents b. Hydrocarbons c. Drowning (fresh, salt water) 5. Drugs a. Narcotic drugs b. Chemotherapy agents c. Nitrofurantoin d. Amiodarone 6. Inhalational injuries a. Toxic chemicals (chlorine, fluorine, etc.) b. Oxygen c. Oxides of nitrogen d. Smoke 7. Metabolic conditions a. Pancreatitis b. Severe uremia c. Paraquat, acetaminophen ingestion 8. Radiation |
Epidemiology and Risk Factors
An accurate estimation of the incidence of ARDS depends on an accurate definition. Previous studies have found incidences between 1.5 and 13.5 per 100,014 population, although rates of 75 per 100,014 were estimated by the National Institutes of Health in the United States (discussed elsewhere13,14) Using the 1994 consensus definition, Scandinavia has reported a 17.9 per 100,014 rate for acute lung injury, whereas the incidence of ARDS was 13.5 per 100,014.15
Shock lung Blast lung Da Nang lung Posttraumatic pulmonary insufficiency Hemorrhagic pulmonary edema Congestive atelectasis Traumatic wet lung Diffuse alveolar damage (DAD) (pathology) |
FIGURE 14–1 Chest radiograph showing diffuse lung infiltrates in a patient with acute adult respiratory distress syndrome (ARDS).
Table 14–1 lists the wide variety of conditions that have been associated with ARDS. These can be divided into two basic groups: those conditions that act as direct pulmonary insults, such as aspiration, toxic inhalations, and lung infections; and those that involve the lung in an indirect fashion, such as shock, massive trauma, and pancreatitis.13,16
Pathophysiology
Alveolar capillary membrane injury is a primary event. The membrane can be injured directly by the initial insult, as in aspiration of gastric fluid or inhalation of noxious chemicals, as well as by a complex interaction of neutrophils and activated complement.17–19 The peripheral blood white cell differential count may show a relative lymphocytosis, indicating neutrophil sequestration in the lung;20,21 this has been confirmed by animal models of ARDS,22–24 which also have demonstrated the necessity of the neutrophil for the development of the syndrome. Neutrophils also liberate superoxide radicals and other reactive oxidant species,25–27 which, in turn, injure endothelial and epithelial cells. This detrimental action can be increased by ventilation with high oxygen concentrations. Neutrophils also secrete a variety of proteases, resulting in destruction of structural proteins, further altering lung structure. Pulmonary macrophages may also have an essential role in the production of damage to the alveolar membrane as they too secrete proteases as well as a variety of ecosanoids. A variety of cytokines and inflammatory mediators have been implicated in ARDS.12,28,29 Tumor necrosis factor (TNF) and many of the interleukin (IL) family, particularly IL-1, have been found to be implicated in the cellular cascade, ultimately promoting and maintaining the inflammatory response (reviewed elsewhere16). This is particularly important in neutrophil recruitment.
The effect of alveolar capillary membrane damage is increased permeability of the endothelium with increased fluid flux from the damaged capillaries into the lung interstitium and airspaces, there forming the characteristic hyaline membrane. This fluid flux can be increased by secondary effects of vasoconstriction, acting to increase hydrostatic pressure. Because the osmotic forces of the blood may also have been diminished by the use of fluid replacements, the Starling forces are shifted toward a net fluid outflow.16
Pulmonary function alterations can be explained on the basis of the increase in interstitial fluid and alveolar collapse due to dilution or inactivation of surfactant.30 Functional residual capacity is decreased because of accumulation of fluid in the interstitial space, resulting in airway closure and distal atelectasis. Lung congestion decreases compliance as does the increase in alveolar surface tension. Hypoxia is a defining feature of ARDS, and is caused by two basic abnormalities. The first, and probably the most important, mechanism is intrapulmonary shunting due to the large numbers of alveoli, which are perfused but not ventilated, whereas the second mechanism involves the mismatch between perfusion and ventilation in those alveoli that continue to be at least partially ventilated.
Pathology
As the term ARDS is indicative of a clinical syndrome, the pathologic terminology (diffuse alveolar damage, DAD) indicates an evolving morphologic process. The pathologic findings of DAD can be divided into three phases:31,32 the acute exudative phase seen up to approximately day 6; the subacute proliferative phase seen between 4 and 10 days; and the chronic fibrotic phase seen after approximately 8 days. In all stages, postmortem findings are striking: the lungs fill the thoracic cavities with no evidence of collapse after the chest wall is opened. In certain conditions (aspiration, radiation), the lesions can be localized to a lobe or segment of a lobe. When the lungs are diffusely affected, they are characteristically heavy, each weighting 1014 g or greater. The sequence of the reaction in DAD appears to be stereotypic; often the lesion cannot be ascribed to any specific etiology.
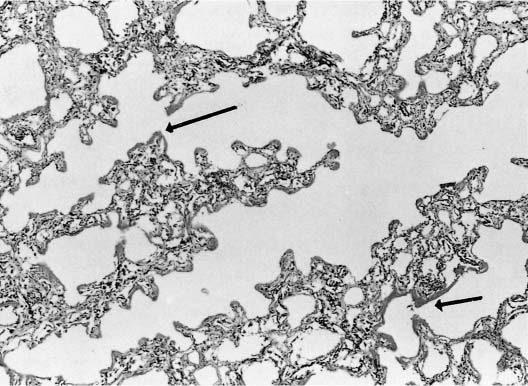
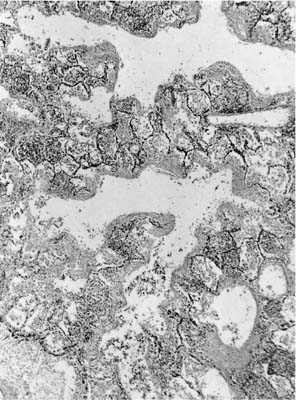
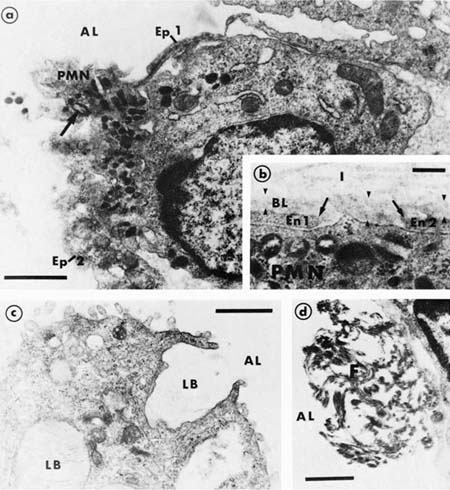
In the acute stage, the lung has a rubbery texture. The fresh-cut surface has a meaty appearance, which, after fixation, shows obliteration of the lung markings, giving a homogeneous appearance (Fig. 14–2). On low-power microscopy, the alveolar ducts and respiratory bronchioles are prominent, with adjacent alveoli collapsed around them (Fig. 14–3). The respiratory bronchioles and alveolar ducts are coated with brightly eosinophilic hyaline membranes (Fig. 14–4), and it is worth stressing that hyaline membranes are not commonly found in the alveoli. The membrane itself is composed of an admixture of fibrin and other plasma proteins. Electron microscopic studies (Fig. 14–5) in this phase show pneumocyte damage and abundant surfactant debris. The earliest phases do not show more than a mild interstitial inflammatory infiltrate, but the interstitium is widened by edema. Alveolar lining cells and capillary endothelial cells can completely slough, leaving their respective basement membranes denuded of cells. Microthrombi are common within the capillaries in acute DAD, and probably form around damaged or sloughed endothelial cells.
As DAD evolves, the gross appearance changes with smooth, firm areas of consolidated lung tissue, and the formation of small cysts. On microscopy, the interstitial infiltrate becomes more marked, with obvious proliferation of fibroblasts, and acute and chronic inflammatory cells fill the airspaces with granulation tissue. Alveolar collapse becomes more marked, and the alveolar ducts increasingly distorted. The hyaline membranes begin to become organized, and both macrophages and fibroblasts are numerous. At this stage, the process may be reversible, and the lung parenchyma may return to normal. In cases of more severe damage, the repair reaction is marked, and tufts of granulation tissue can be seen in the alveoli as well as clusters of macrophages (Fig. 14–6). Thus, the membranes gradually become incorporated into the walls of revised thick-walled airspaces (Fig. 14–7). The pneumocytes are often prominent, with large nucleoli that can be mistaken for viral inclusions, and show increased mitotic activity. Although once considered to be a hallmark of chemotherapeutic toxicity, these cells are entirely nonspecific and are seen in DAD from any etiology (Fig. 14–8). Occasionally, the cytoskeleton is altered dramatically with the formation of Mallory’s hyaline. The newly formed type II cells tend to be closely spaced, forming a row along the alveolar septa (Fig. 14–9). The airspaces can also show bronchiolar or squamous metaplasia (Fig. 14–10), sometimes so atypical as to simulate malignancy.
In the fibrotic stage, the lungs remain heavy, and show larger, still irregular areas of dense consolidation and scattered areas of gray fibrous tissue. As fibrosis progresses, the pleural surface may show the cobblestoning characteristic of underlying fibrosis. In some cases ARDS can be organized in the same fashion as seen in infants,33,34 producing radiographically hyperinflated lungs with cyst formation, a condition known in both as bronchopulmonary dysplasia (BPD). Grossly, the lungs are fibrotic with reorganized airspaces and cyst formation (Fig. 14–11). These cysts measure between 1 mm and several centimeters, and can be seen throughout the affected lung, but are most marked in the lung periphery. The cysts of BPD appear to be centered around alveolar ducts, and consist of irregular, inflamed, and densely fibrotic walls with collapse of the adjacent alveoli (Figs. 14–12 and 14–13). Within the scarred areas, the pulmonary vessels show thickened distorted walls with intimal fibrosis and narrowing or actual obliteration of their lumina. Patients who develop this syndrome tend to have required high-pressure ventilation with increased oxygen concentrations.35
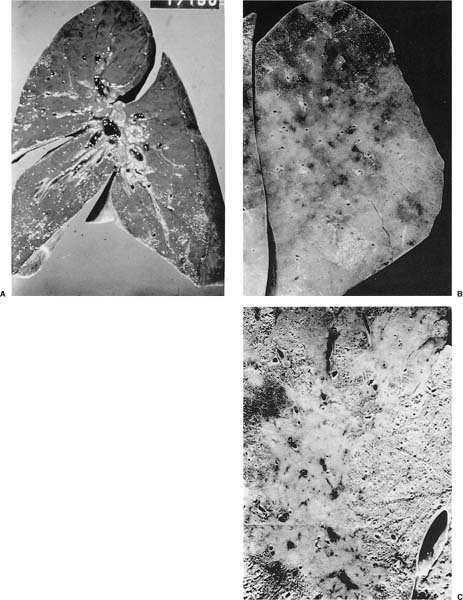
FIGURE 14–2 Gross specimens from patients with acute ARDS. (A) The lungs were heavy and had a meaty texture. (B,C) There is diffuse loss of the lung markings. (C) An example of localized ARDS, secondary to aspiration.
FIGURE 14–3 Photomicrograph from a patient with acute ARDS. Note the eosinophilic hyaline membranes (arrows) lining the alveolar ducts.
Pathologic Differential Diagnosis
Histologic findings must always be interpreted in conjunction with the clinical findings, and failure to do so explains many of the misconceptions present in the older literature.
Idiopathic Interstitial Fibrosis (Usual Interstitial Pneumonia, Fibrosing Alveolitis)
It can be very difficult, and often impossible to discover the etiology of a fibrotic lung (see Chapter 20). Grossly, usual interstitial pneumonia (UIP) tends to spare the central aspects of the lung and has more severe peripheral fibrosis, whereas DAD affects the whole lung or lung lobe. Peripheral cyst formation is a hallmark of UIP, whereas cysts are less numerous and more irregularly distributed in organized DAD. On microscopy, the fibrotic walls in the airspaces in UIP are formed of dense bands of collagen with small foci of fibroblastic proliferation compared with the more poorly collagenized fibrous tissue in DAD with its obvious and diffuse fibroblastic proliferation. Although exacerbations of UIP can have hyaline membranes, these tend to occur within the honeycombed areas, providing a combination of obvious dense collagen and the superimposed acute inflammation with hyaline membranes. Clinically, the diseases are very distinct, UIP having a history of months to years, whereas DAD has the short-term history of ARDS described earlier. Some of the cases described by Hamman and Rich7 appear to have been cases of ARDS,36 as do many of the cases in the pneumoconiosis literature.9 The entity of acute interstitial pneumonia8 has the clinical and pathologic features of ARDS, but does not have a recognizable etiology; it is possible that at least some of the above cases represent acute interstitial pneumonia.
FIGURE 14–4 Higher power magnification of hyaline membranes from a case of acute ARDS. In the acute phase, they are generally acellular and brightly eosinophilic.
FIGURE 14–5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree