, Richard R. Liberthson2, Richard R. Liberthson3, Ami B. Bhatt4 and Ami B. Bhatt5
(1)
Harvard Medical School Adult Congenital Heart Disease Program, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
(2)
Harvard Medical School, Boston, USA
(3)
Adult Congenital Heart Disease Program, Cardiology Division, Department of Medicine and Pediatrics, Massachusetts General Hospital, Boston, MA, USA
(4)
Harvard Medical School, Boston, USA
(5)
Adult Congenital Heart Disease Program, Cardiology Division, Department of Medicine, Massachusetts General Hospital, Boston, MA, USA
Abstract
The population of adults with congenital heart disease (CHD) is rapidly growing and presently exceeds one million individuals in the United States. It is a heterogeneous patient group comprising those with previously unrecognized and untreated lesions, as well as many prior palliated or corrective interventions. Most congenital heart disease patients benefit from ongoing cardiac supervision and a substantial number require highly specialized management and further intervention [1].
Abbreviations
ACE
Angiotensin converting enzyme
ADHD
Adult Congenital Heart Disease
ALCAPA
Anomalous left coronary artery origin from the PA
AR
Aortic regurgitation
AS
Aortic stenosis
ASD
Atrial septal defect
AV
Atrioventricular
BAV
Bicuspid Aortic Valve
BP
Blood pressure
CC-TGA
Congenitally corrected Transposition of the Great Arteries
CHD
Congenital heart disease
CTA
CT angiography
CXR
Chest X Ray
EF
Ejection fraction
ICD
Implantable cardioverter defibrillator
LA
Left atrium
LAD
Left anterior descending
LBBB
Left bundle branch block
LV
Left ventricular
LVOT
Left ventricular outflow tract
MI
Myocardial infarction
MR
Mitral regurgitation
MV
Mitral valve
NO
Nitric oxide
PA
Pulmonary artery
PDA
Patent ductus arteriosus
PFO
Patent foramen ovale
PR
Pulmonic regurgitation
PS
Pulmonic stenosis
PVR
Pulmonary vascular resistance
RA
Right atrium
RBBB
Right bundle branch block
RCA
Right coronary artery
RV
Right ventricle
RVH
Right ventricular hypertertrophy
RVOT
Right ventricular outflow tract
SVC
Superior vena cava
SVT
Supraventricular tachycardia
TEE
Transesophageal echocardiogram
TGA
Transposition of the Great Arteries
TOF
Tetralogy of Fallot
TR
Tricuspid regurgitation
TV
Tricuspid valve
VSD
Ventricular septal defect
VT
Ventricular tachycardia
WPW
Wolff-Parkinson-White syndrome
Background
The population of adults with congenital heart disease (CHD) is rapidly growing and presently exceeds one million individuals in the United States. It is a heterogeneous patient group comprising those with previously unrecognized and untreated lesions, as well as many prior palliated or corrective interventions. Most CHD patients benefit from ongoing cardiac supervision and a substantial number require highly specialized management and further intervention [1].
1.
Left-to-Right Shunt Lesions
2.
Obstructive Lesions
3.
Complex Lesions
Left-To-Right Shunt Lesions
Atrial septal defect (ASD)
Epidemiology:
Most common congenital heart lesion in adults, female predominance
Rule out Holt Oram syndrome: autosomal dominant, congenital abnormality of the hand and radius, families should be screened
Types (Fig. 21-1a):
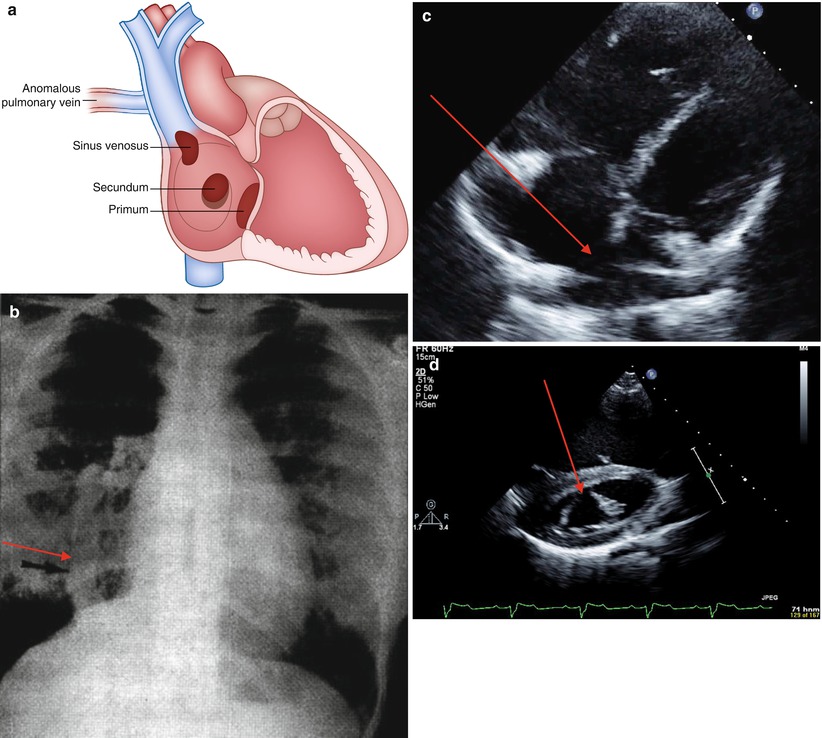
FIGURE 21-1
Atrial septal defects. (a) Depiction of types of atrial septal defects. (b) CXR of patient with Scimitar syndrome. Anomalous pulmonary venous drainage to the IVC (arrow). (c) Four chamber view with drop out of the intraatrial septum consistent with secundum ASD (arrow). (d) Parasternal short axis view, cleft mitral valve often associated with primum ASD (arrow)
Secundum (65 %): varies in size, usually isolated lesion, mitral regurgitation (MR) occurs in elderly
Primum (10–15 %): often large and associated with cleft anterior mitral leaflet with MR
Sinus venosus (10–15 %): commonly associated with partial anomalous pulmonary venous drainage (superior sinus venosus defect – right upper pulmonary venous anomaly; inferior sinus venosus defect- right lower pulmonary venous anomaly: Scimitar)
Coronary sinus septal defects: rare, associated with complex cardiac lesions
Clinical Presentation: ASD presents with a volume load to the right atrium (RA) and right ventricle (RV)
Varies from asymptomatic (incidentally found), to progressive RA and RV dilation with larger defects causing supraventricular tachyarrhythmia, fatigue, exercise intolerance.
In older individuals (age >60): atrial fibrillation, and with a significant shunt, right heart dilation and hypocontractility. Left-to-right shunt may increase with advancing age as left ventricular (LV) compliance worsens, or systemic hypertension, MR, or LV disease develops
Right ventricular hypertension may lead to right –to-left shunting, hypoxemia, cyanosis and rarely paradoxical embolus.
Reversible flow-related pulmonary hypertension is common with large defects in older patients; irreversible pulmonary vascular obstruction is less common (Eisenmenger syndrome).
Physical exam:
Wide and fixed splitting of the second heart sound
Soft systolic pulmonary flow murmur
Precordial lift (RV enlargement)
ECG findings:
Incomplete right bundle branch block (RBBB), may progress to complete with larger shunts and age.
Primum defects typically have left axis deviation, sinus venosus and secundum generally have a rightward axis.
Sinus venosus may have a low ectopic atrial rhythm (negative p waves in leads II, III, aVF).
Chest X Ray (CXR): may reveal the curvilinear shadow of an anomalous pulmonary vein (with inferior sinus venosus defects, Scimitar sign Fig. 21-1b)
Echo findings:
Right heart enlargement.
Atrial septal drop out (Fig. 21-1c).
Main pulmonary artery (PA) enlargement and increased transpulmonic flow.
In primum ASD, evaluation for mitral valve (MV) anterior leaflet cleft (Fig. 21-1d) and MR is important, rule out caval type ventricular septal defect (VSD).
In secundum ASD, a transesophageal echocardiogram (TEE) defines location and anatomy to determine candidacy for device closure.
Diagnosis of sinus venosus defect difficult with 2D echo and usually requires TEE.
CT/MR: May be used for sinus venosus defect and partial vein imaging if not seen on echocardiogram.
Cardiac catheterization: hemodynamic assessment of pulmonary vascular resistance (PVR) and reversibility (response to pulmonary vasodilator therapy: 100 % O2, nitric oxide [NO]), and shunt calculation is essential to determine closure candidacy. In some, test balloon occlusion in the catheterization lab may facilitate decision process.
Management:
ACC/AHA Class I recommendation: closure of an ASD either percutaneously or surgically is indicated for RA or RV enlargement with or without symptoms in predominantly left-to-right shunts, or bidirectional shunting through a larger ASD with low or responsive PVR [2].
ACC/AHA Class III recommendation: closure not indicated if severe irreversible pulmonary hypertension (Eisenmenger physiology) [2].
Percutaneous closure: uncomplicated secundum defects with appropriate anatomy [3]
Surgical closure: large secundum ASDs, unusual anatomy, and all sinus venosus, primum ASDs and coronary sinus defects; Pre-operative imaging will define anomalous pulmonary venous drainage and MV abnormalities that may also require repair. For sinus venosus defects with anomalous pulmonary venous drainage, a Warden technique is sometimes used.
Post-operative complications: residual shunt, MR and atrioventricular (AV) conduction abnormality (rare, and all more likely with primum ASD repair). Sinus venosus surgical complications include sinus node dysfunction/supraventricular tachycardia (SVT), pulmonary venous obstruction at anastomosis site, and rarely superior vena cava (SVC) obstruction. Typically, RV size and function improve post operatively even in advanced age.
Pregnancy and delivery: well tolerated in most patients. Ideal to discuss and repair if needed preconception. With large bidirectional shunts, IV filters are recommended.
Ventricular septal defects (VSD)
Epidemiology: among most common congenital heart entities in early childhood, 2/3 close by early school age; larger VSDs present volume burden to the left atrium (LA) and LV and in some, the RV
Types (Fig. 21-2a):
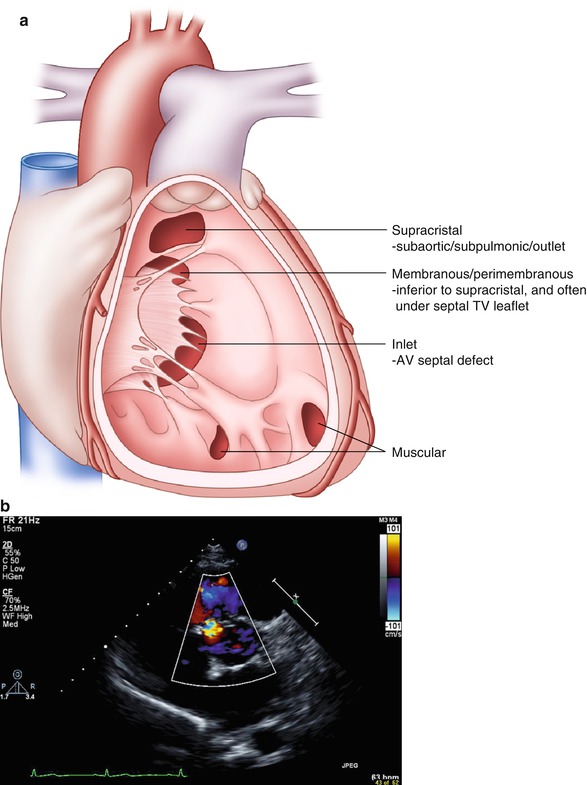
FIGURE 21-2
Ventricular septal defects. (a) Depiction of types of VSDs. (b) Parasternal short axis view of perimembraneous VSD, systolic flow noted around 10’o clock
Perimembranous: 60–70 %
Muscular: singular or multiple (10 %), in adults generally small and restrictive
Supracristal: (5 %) more common in Asian populations, usually small defects located beneath the aortic annulus, may lead to progressive aortic leaflet prolapse and insufficiency (right and noncoronary cusp); typically asymptomatic until aortic regurgitation (AR) is severe.
AV canal defect: common in Down’s syndrome, typically involves anterior mitral cleft and occasionally cleft tricuspid septal leaflet; primum ASD may coexist
Clinical presentation: varies depending on prior management
If small isolated restrictive defect, typically asymptomatic with a murmur
If well repaired earlier, typically asymptomatic. Residual VSD is usually small, heart block may occur, and residual or recurrent AR (in a supracristal VSD) or MR (in AV canal VSD)
If large defect uncorrected in childhood → Eisenmenger syndrome
ECG: typically RBBB (pre or post repair); marked left axis deviation (and sometimes AV block) with AV canal VSDs, right axis deviation/right ventricular hypertrophy (RVH) if significant pulmonary hypertension and in rare patients with progressive RV infundibular hypertrophy.
Physical exam:
Classic small restrictive VSD murmur is holosystolic, loud and harsh, augments with isometrics.
Associated diastolic murmur of AR if aortic cusp prolapse is present
In AV Canal patients, an MR or tricuspid regurgitation (TR) murmur from a cleft valve may also be appreciated
If prior pulmonary banding (previously done in infancy to avoid pulmonary volume overload until corrective repair could be undertaken), a loud systolic ejection murmur of supravalvular pulmonic stenosis can be appreciated, and if RVH, there may be a jugular venous a wave on exam
Eisenmenger exam-see below
Echocardiography:
Define detailed VSD anatomy (Fig. 21-2b), estimate RV pressure and gradient across the defect(s), and identify associated lesions
If corrected, evaluate for residual shunt and assess RV pressure and rule out associated lesions
Catheterization: performed pre-operatively for pulmonary vascular assessment, coronary screening in older patients, and define associated lesions.
Management:
Small, restrictive lesions rarely require specific management
ACC/AHA Class I recommendation: closure of a VSD is indicated when there is a Qp/Qs (pulmonary-to-systemic blood flow ratio) of 2.0 or more and clinical evidence of LV volume overload, OR if a patients has had a history of endocarditis [2]
ACC/AHA Class III recommendation: closure of a VSD is not recommended in patients with severe irreversible pulmonary hypertension [2]
Role for percutaneous approach is evolving for VSDs remote from the tricuspid valve (TV) and aorta
Complications:
Endocarditis
Potential right-to-left thrombotic complication avoid intracardiac RV pacer or implantable cardioverter defibrillator (ICD) wires
Progressive aortic cusp prolapse and insufficiency, and rarely sinus of Valsalva aneurysm or fistula (fistula will result in continuous murmur)
Large VSDs left untreated may lead to increased PVR from long term increased pulmonary flow, and reversal of the shunt (Eisenmenger syndrome).
Heart block is an occasional early or late post operative complication
Patent ductus arteriosus (PDA)
General: PDA is essential for prenatal survival. It typically closes early in infancy, with a higher incidence of PDA in premature infants and those living at high altitudes. Commonly associated with Congenital Rubella Syndrome as is branch pulmonic stenosis.
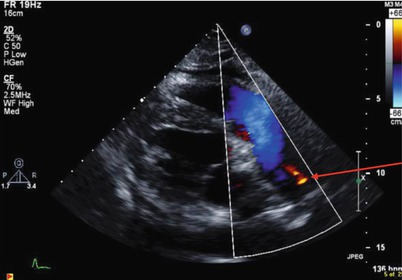
FIGURE 21-3
Patent ductus arteriosus: modified parasternal view with laminar flow across the pulmonic valve leaflets and main PA, and PDA flow from the aorta into the LPA (red arrow)
Clinical Presentation: varies according to size, from asymptomatic (incidentally noted), to LA and LV volume overload or Eisenmenger syndrome when large and unrepaired
Physical Exam:
Small PDA: soft continuous infraclavicular, left sternal border or left upper back murmur, enhanced with isometrics (Fig. 21-3)
Moderate PDA: ventricular enlargement and a displaced point of maximal impulse on palpation
Large PDA: LA and LV enlargement; If pulmonary hypertension, there will be a prominent pulmonic component to the second heart sound and RV heave with cyanosis and clubbing [Eisenmenger individual: may have differential cyanosis: cyanosis of feet (clubbing of toes) and perhaps left hand with normal right hand pulse oximetry]
Differential for continuous murmur includes: PDA, coronary AV fistula, aortopulmonary window, pulmonary AV malformation (Peutz Jaeger syndrome) or the systolic/diastolic murmur of aortic stenosis (AS)/AR
Complications: endarteritis, left heart failure, pulmonary vascular disease if large and unrepaired; in older adults calcification, aneurysm and dissection risk which may complicate repair
Management:
ACC/AHA Class I recommendation: closure of PDA either percutaneously or surgically is indicated if there is LA and or LV enlargement, if pulmonary hypertension is present in the presence of net left-to-right shunting OR prior endarteritis. Surgical closure is most appropriate when PDA is too large for percutaneous closure device, or if PDA is aneurysmal, or there is endarteritis [2]
ACC/AHA Class III recommendations: closure is not indicated if pulmonary hypertension with net right-to-left shunt [2]
Sinus of Valsalva fistula:
Description: Typically arise from the right or noncoronary sinus of Valsalva and enter the right heart. May be associated with a VSD high in the basal septum; Commonly associated with connective tissue abnormality
Clinical presentation: new onset prominent diastolic or continuous murmur, occasionally precipitated by strenuous isometric exertion
Management:
Endocarditis and AR risks exist
Surgical repair; transcatheter occlusion for selected patients
Recurrence may occur
Obstructive Lesions
Left Ventricular Outflow Tract (LVOT) Obstruction
Congenital Aortic Valvular Stenosis:
Bicuspid Aortic Valve (BAV):
Epidemiology: most common congenital heart lesion, male predominance, estimated 1–2 % of population, may be familial, multiple morphologic variants, may be undiagnosed for many years
Can be associated with aortic coarctation, should be ruled out in Turner’s Syndrome
Important association with medial connective tissue abnormalities of the ascending aorta
Abnormalities of smooth muscle, extracellular matrix, elastin and collagen of the ascending aorta sometimes result in progressive dilation and increase dissection risk with age
Ascending aortic dilation does not correlate with valve stenosis severity
Clinical presentation and physical exam: varies with severity of stenosis or regurgitation
Asymptomatic evident by only soft systolic flow murmur and early systolic ejection sound (uncommon after age 40 years)
Severe LV outflow obstruction, syncope, chest pain, heart failure and endocarditis
Stenosis may be progressive in mid life as well as with advanced age and renal dysfunction
Regurgitation is less common than stenosis
Echocardiography:
Mean Doppler gradients correlate well with transcatheter pull back gradients
Important to assess ascending aortic dimensions serially (frequency of imaging varies with size of aorta at initial assessment: if <40 mm → every 2 years, if ≥40 mm → annually or more frequently if rapid change or new symptoms) even in previously operated aortic valve patients who have not had prior ascending aortic intervention. CT angiography (CTA) is preferable as aortic size approaches surgical dimensions (see below).
Intervention: transcatheter balloon dilation may be appropriate in younger adults with severe stenosis without significant AR, otherwise, surgical valvuloplasty or valve replacement per valve guidelines.
ACC/AHA Class I recommendation: aortic surgical intervention is indicated in a patient with a BAV and ascending aorta is 5.0 cm or more, or if there is progressive dilatation at a rate greater than 5 mm per year [2]
Unicuspid aortic valve: rare, may present with stenosis or regurgitation. It may be associated with ascending aortic dilation. Transcatheter balloon dilation may cause AR, therefore surgical intervention for severe obstruction or insufficiency is recommended.
Quadricuspid aortic valve: very rare. Presents typically late in life with AR requiring aortic valve replacement, stenosis is rare.
Discrete subaortic membrane:
General:
Congenital or acquired, (occasionally associated with primum ASD, double chamber RV, or tetralogy of Fallot [TOF])
Prevalence among patients with ACHD is approximately 6.5 %.
Membranes vary in thickness, morphology and distance below the aortic valve (Fig. 21-4), AR is common due to high velocity flow jet causing aortic valve sclerosis
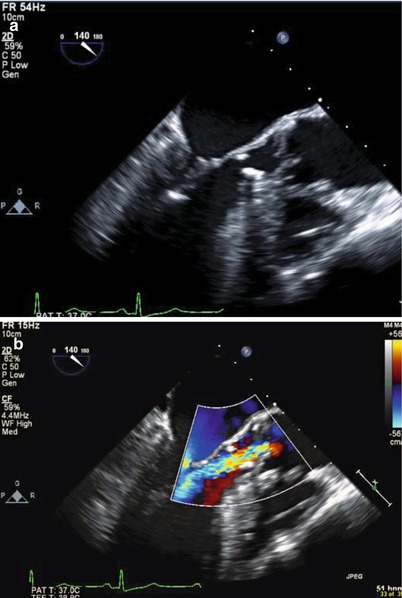
FIGURE 21-4
Subaortic membrane: (a) Transespophageal echocardiogram, membrane noted approximately 1.2 cm below the aortic valve along the anterior surface of the ventricular septum, as well as the anterior aspect of the mitral valve. Note the aortic valve leaflets appears thickened and degenerated. (b) Associated aortic insufficiency
Familial occurrence approaches 15 % among primary relatives (who should be screened)
Bacterial endocarditis occurs
Infrequent ascending aortic dilation
Physical exam:
Systolic crescendo decrescendo murmur and absence of ejection sound
AR murmur (more than 50 %)
Management:
Percutaneous balloon dilation is rarely successful
Surgical resection for significant obstruction or insufficiency, aortic valve should be evaluated at the time of surgery as well.
ACC/AHA Class I recommendation: Surgical intervention indicated with peak gradient of 50 mmHg or mean gradient of 30 mmHg by echocardiography OR for lesser gradients if progressive aortic regurgitation and LV dilation (end diastolic diameter of 50 mm or more or LV ejection fraction less than 55 %) [2] – however each case should be considered independently as significant heterogeneity exists.
Post operative issues:
Residual hypertrophic LVOT obstruction, AR, left bundle branch block (LBBB) or surgical VSD can occur
Membranes may recur post operatively (15 %)
Supravalvular Aortic Stenosis: Ascending aortic narrowing (Fig. 21-5 ) commonly associated with Williams Syndrome. Obstruction may be discrete or diffuse, may extend variably cephalad in the ascending aorta and arch. Generally spares the coronary arteries and aortic valve. Surgical revision when obstruction is significant. Differential diagnosis includes: Takayasu and rarely homozygous hypercholesterolemia with secondary lipomatous deposition in the proximal ascending aorta.
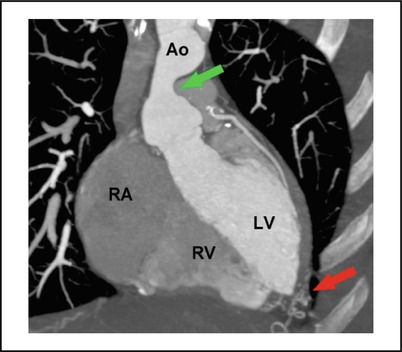
FIGURE 21-5
Supravalvular aortic stenosis (green arrow) in a patient with William syndrome, CT angiography, red arrow depicts collateral vessels (Sidhu MS et al. MGH cardiovascular imaging 2011)
Coarctation of the aorta:Get Clinical Tree app for offline access
Definition/anatomy:
Typically focal narrowing in the region of the ligamentum arteriosum adjacent to the origin of the left subclavian artery (Fig. 21-6a)
< div class='tao-gold-member'>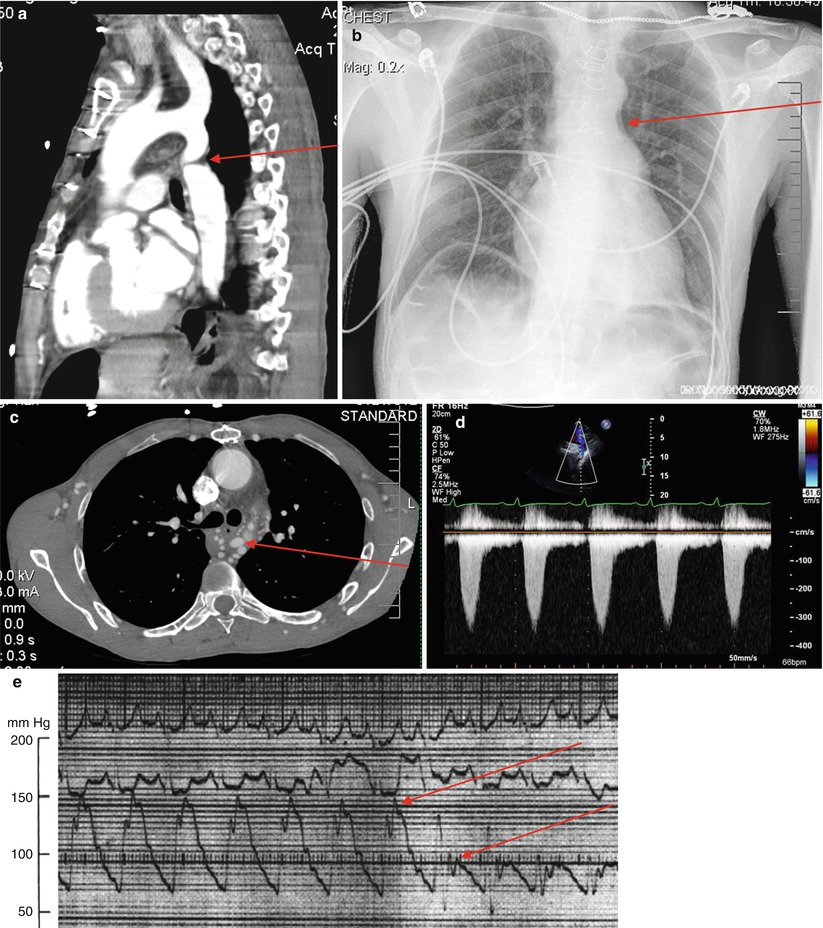 Only gold members can continue reading. Log In or Register to continue
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


