Adenocarcinoma, Nonmucinous
Allen P. Burke, M.D.
Fabio R. Tavora, M.D., Ph.D.
Haresh Mani, M.D.
Classification
The majority of adenocarcinomas of the lung are derived from cells localized to the bronchoalveolar duct junction that differentiate to Clara cells or pneumocytes.1 Expression profiles have classified adenocarcinomas into so-called terminal respiratory unit type and nonterminal respiratory type, roughly corresponding to nonmucinous and mucinous adenocarcinomas, respectively.2 TTF-1 expression is localized to pneumocytes and terminal respiratory cells and is the hallmark of nonmucinous adenocarcinomas, although a small proportion of nonmucinous tumors cannot express this antigen and are of uncertain histogenesis.
Nonmucinous adenocarcinomas are further subclassified based on growth patterns; the predominant type is used as a modifier, and the tumor checklist should provide an estimate as to the proportions (in percentages) of the other subtypes present.
Clinical and Incidence
The overall proportion of lung cancers that are adenocarcinomas is increasing and is now over 40%, with squamous and small cell constituting the majority of the remainder.3 There is no longer a male predilection, as the rates of smoking are no longer gender biased. Adenocarcinomas represent the largest group of carcinomas in patients who have never smoked, now constituting over 10% of cases.4 Lung cancer in the never smokers affects women more often than men and is more likely to harbor epidermal growth factor receptor (EGFR) mutations.
Radiologic Findings
Invasive adenocarcinomas are typically peripheral nodules, which have irregular, spiculated borders. Cavitation, as is commonly seen in squamous carcinomas, does not occur. Those tumors with an in situ (lepidic) growth pattern will show a corresponding ground-glass component on high-resolution computed tomography scans.5 Similar to histopathologic assessment, solid tumors by computed tomographic scans show a significantly greater rate of lymphatic, vascular, and pleural invasion and lymph node metastasis compared with tumors with ground-glass (in situ) component.6
Tissue Sampling
Adenocarcinomas of the lung are often diagnosed on cytologic samples or small biopsies. Patients with advanced disease are usually treated without resection; therefore, proper diagnosis of these samples is critical to treat advanced stage patients, and a nonspecific diagnosis such as “non-small cell lung cancer” is not sufficient (see Chapter 72). Features important to note on wedge biopsies, segmentectomies, or lobectomies include invasion into the pleura, distance of tumor from margin, and size of invasive tumor. Wedge biopsy may be performed for intraoperative confirmation of malignancy and invasion >5 mm, by frozen section, after which completion lobectomy is performed. Mediastinoscopy is often performed prior to wedge resection if there are enlarged level II or level III lymph nodes, which will be sent for frozen section to rule out metastatic disease. In general, disease spread to these nodes will preclude surgery for the primary tumor, and the surgery will be terminated.
Peripheral adenocarcinomas may occasionally be treated with neoadjuvant chemoradiation prior to tumor resection. In these cases, it is important to state the degree of treatment effect, preferably as a percentage tumor necrosis or fibrosis.
Gross Findings
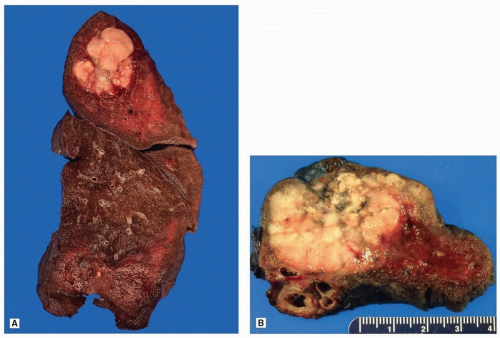
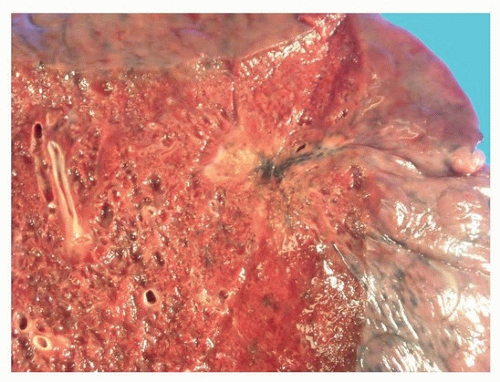
Adenocarcinomas of the lung are usually peripheral nodules with irregular borders (Fig. 75.1), which often have central scarring, which may retract the pleural surface (Fig. 75.2).
Microscopic Findings
Well-differentiated adenocarcinomas generally have a peripheral in situ (lepidic) component and typically have a central scar that is rich in elastic tissue (Fig. 75.3). Poorly differentiated tumors are less likely to have abundant fibrosis and more likely to show pleural, lymphatic, and large-vessel invasion. A desmoplastic reaction to tumor invasion occurs occasionally, but is less common than squamous carcinomas. Necrosis is not uncommon in higher-grade tumors, but central cavitation, as occurs in squamous carcinoma, is exceptionally rare.
There are five predominant histologic patterns of nonmucinous adenocarcinomas of the lung: in situ (lepidic), acinar, papillary, micropapillary, and solid. The cribriform pattern is usually considered
a subset of the solid pattern, but is sometimes separated out, as it has a prognosis intermediate between solid and acinar. The tumor is generally diagnosed by the predominant pattern, and other types described as percent growth. The frequencies of the individual tumor patterns vary by series and country, with papillary carcinoma common in Japan.7 Otherwise, most series place acinar predominant as the most common type, followed by solid, lepidic predominant, and papillary, with micropapillary having a relatively wide range, depending on the series.8,9,10
a subset of the solid pattern, but is sometimes separated out, as it has a prognosis intermediate between solid and acinar. The tumor is generally diagnosed by the predominant pattern, and other types described as percent growth. The frequencies of the individual tumor patterns vary by series and country, with papillary carcinoma common in Japan.7 Otherwise, most series place acinar predominant as the most common type, followed by solid, lepidic predominant, and papillary, with micropapillary having a relatively wide range, depending on the series.8,9,10
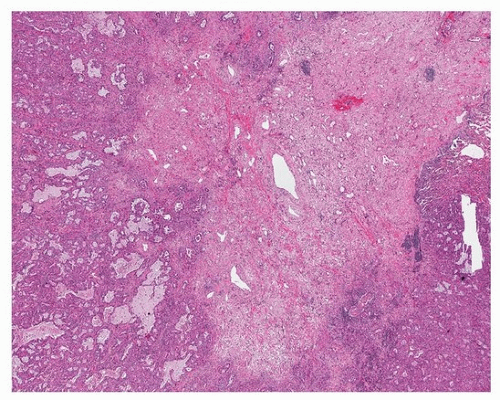 FIGURE 75.3 ▲ Peripheral adenocarcinoma. The scar is formed largely of grayish-blue elastotic fibrosis. |
It is well known that over 90% of lung adenocarcinomas have more than one pattern (hence the abandonment of the term “mixed” subtype, which was previously applied to most tumors). In one series, 93% were mixed with >1 pattern, 46% had >2 patterns, 20% had >3 patterns, and 3% had >4 patterns.10 Tumors with solid growth have a higher proportion of this pattern overall, with micropapillary pattern usually representing a relatively small portion of the tumor.
Vascular invasion may manifest as lymphatic, arterial, and venous invasion. Relatively recently, spread through alveolar spaces (tumor islands, or intra-alveolar tumor nests) has been recognized as a type of invasion.11
An alternative classification of lung adenocarcinomas considers lepidic and acinar as “alveolar,” retains a papillary classification, and refers to solid and other types of invasive growth as “destructive.”12
Lepidic Predominant
Because all lung adenocarcinomas are designated based on the predominant pattern, the term “lepidic predominant” is synonymous with “lepidic adenocarcinoma,” and the terms are used interchangeably.10,13 “Predominant” generally signifies >50% of the tumor, although some have used the percent surrounding edge of the adenocarcinoma with in situ growth pattern.14 The histologic features of this pattern are identical with nonmucinous adenocarcinoma in situ or the lepidic areas in minimally invasive adenocarcinoma (see previous chapter).
The invasive areas of lepidic-predominant tumors usually have extensive areas of acinar growth. The border separating the invasive and noninvasive (in situ) areas may be elusive and difficult to pinpoint.14 The distinction between acinar and lepidic is based on absence of underlying alveolar architecture in the former and the recognition of intact alveolar septa in the latter. Less commonly, the invasive components are higher grade, such as micropapillary, solid, and cribriform.
Acinar
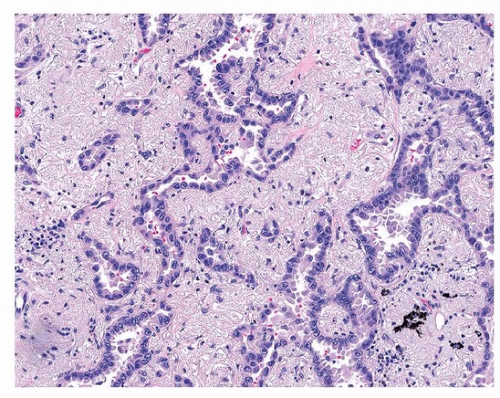
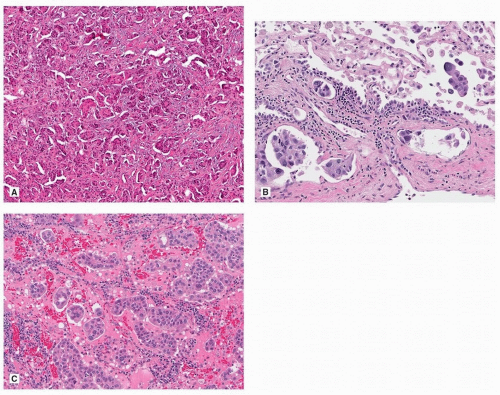
Acinar adenocarcinoma is composed of glands or tubules that are either closely packed (Fig. 75.4) or separated by fibrous or elastotic stroma (Fig. 75.5). Tumor cells with prominent snouts that resemble Clara cells are common in well-differentiated tumors (Fig. 75.6). Acinar adenocarcinomas with higher cytoplasmic grade (mitotic activity, nuclear pleomorphism) are more likely to have invasive cribriform or solid areas, as well as vascular invasion.
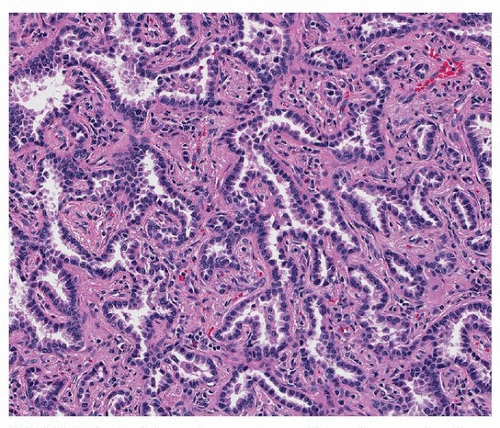 FIGURE 75.4 ▲ Acinar adenocarcinoma. This pattern may be difficult to distinguish from adenocarcinoma in situ, but an intact alveolar architecture is absent. |
Papillary
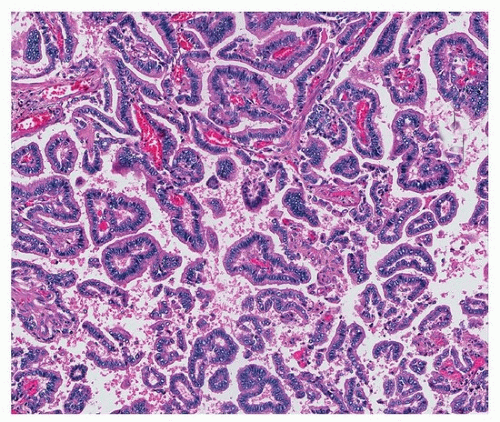
Classic papillary adenocarcinomas are composed of fibrovascular cores lined by columnar or cuboidal cells, sometimes with apical snouts (“Clara cell” morphology) (Fig. 75.7). Often, there is a combination of acinar and papillary growth, with smaller papillary projections within somewhat cystically dilated acini. As with other types, pure papillary tumors are uncommon and often accompanied by acinar or micropapillary areas.
Cribriform
Cribriform adenocarcinoma ranges from fairly large cystic spaces within dilated acini to patterns that merge with solid, having only
inconspicuous glandular areas (Fig. 75.8). When there is “dirty necrosis” within the irregular and variously sized cribriform glands, the term “enteric” is often applied, especially when the cribriform spaces have an appearance of garlands (a term seemingly reserved for enteric adenocarcinoma in this setting). Enteric carcinoma is discussed further in Chapter 77.
inconspicuous glandular areas (Fig. 75.8). When there is “dirty necrosis” within the irregular and variously sized cribriform glands, the term “enteric” is often applied, especially when the cribriform spaces have an appearance of garlands (a term seemingly reserved for enteric adenocarcinoma in this setting). Enteric carcinoma is discussed further in Chapter 77.
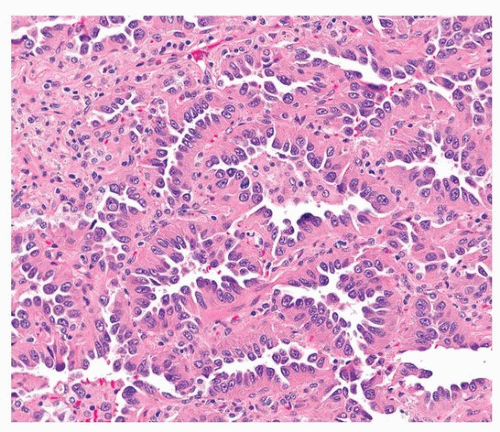 FIGURE 75.6 ▲ Acinar adenocarcinoma. There is minimal scarring. The tumor cells have a characteristic hobnail appearance (Clara cell differentiation). |
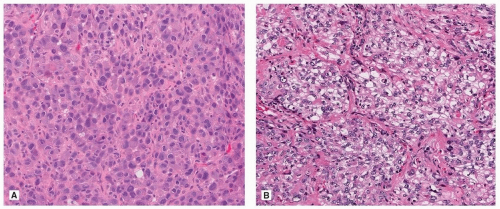
Solid
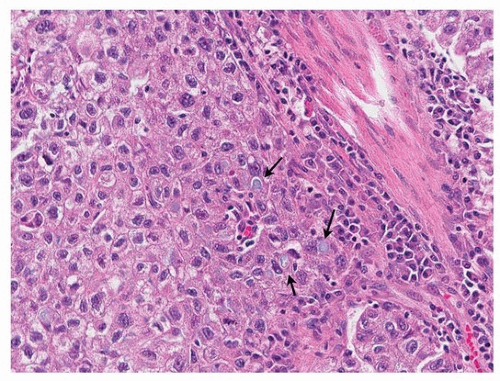
Like cribriform adenocarcinoma, solid adenocarcinoma is also a heterogeneous group, ranging on one end to tumors that show no evidence of differentiation other than TTF-1 positivity, to distinct cribriform adenocarcinoma (Fig. 75.9). When mucin vacuoles are evident by light microscopy or mucin stains (mucicarmine or periodic acid-Schiff [PSA]), then adenocarcinoma can be diagnosed without immunohistochemical stains (Fig. 75.10). Somewhat arbitrarily, it has been recommended that intracellular mucin should be present in at least five tumor cells in each of two high-power fields, seen on PAS or mucicarmine stains, if there are no clear glandular areas.15
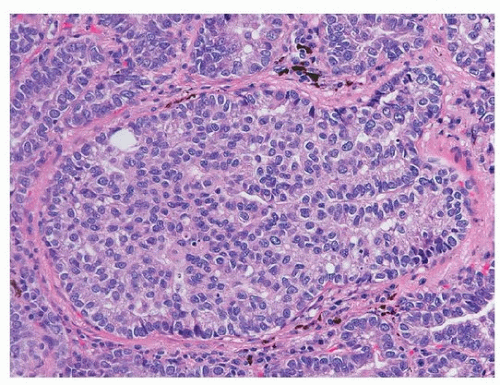 FIGURE 75.8 ▲ Adenocarcinoma, solid type. Adenocarcinoma with cribriforming is generally considered a solid subtype. |
Micropapillary
Micropapillary adenocarcinoma is similar to that seen in other organs, such as the bladder, breast, and ovary. The papillary tufts form florets that lack fibrovascular cores, resulting in inverted polarity, with mucinous brush border along the membrane segment facing the outer periphery of the papillary cluster. Micropapillary adenocarcinoma may occur within stroma (stromal invasion), lymphatics, or alveolar spaces (Fig. 75.11).16 With aerogenous spread, the clusters appear detached in the alveolar spaces as rings and cribriform glandular structures. In all three patterns, the tumor cells are small and cuboidal with minimal nuclear atypia, and lymphatic invasion is frequent. Psammoma bodies may be present.
Tumor Islands
The presence of tumor islands (also known as “spread through alveolar spaces,”11 “aerogenous spread,”16 and “small cluster invasion”)17 is typically associated with micropapillary adenocarcinoma. They have also been described in solid and mucinous adenocarcinomas and infrequently in lepidic or acinar growth patterns. Tumor islands have been associated with smokers and higher nuclear grade.18
Immunohistochemical Findings
Thyroid Transcription Factor-1
Thyroid transcription factor-1 (TTF-1) is the most frequently applied immunohistochemical marker for nonmucinous adenocarcinoma and has even been found to have prognostic significance.19 There are many reports of the sensitivity and specificity of this marker, with a range of results. A study of tissue microarrays demonstrated a sensitivity of 86%,20 very similar to a sensitivity of 88% in resected specimens.21 These values are higher than a recent meta-analysis showing a sensitivity (combined with napsin-A) of only 76%.22 Importantly, the subtype of adenocarcinoma affects the rate of TTF-1 positivity
(Table 75.1). Virtually all nonmucinous lepidic-predominant and papillary adenocarcinomas are TTF-1 positive, compared to 94% of acinar-predominant adenocarcinoma, 93% of patients with micropapillary-predominant adenocarcinoma, and 86% of solid-predominant adenocarcinoma.19 Another study found that in solid adenocarcinomas, any nuclear TTF-1 expression was present in 69% of tumors.23 The subgroup with the lowest rate (50% to 60%) is mucinous adenocarcinoma (see subsequent Chapter 76). TTF-1 positivity is most helpful in differential diagnosis of tumors with a solid growth pattern (Fig. 75.12).
(Table 75.1). Virtually all nonmucinous lepidic-predominant and papillary adenocarcinomas are TTF-1 positive, compared to 94% of acinar-predominant adenocarcinoma, 93% of patients with micropapillary-predominant adenocarcinoma, and 86% of solid-predominant adenocarcinoma.19 Another study found that in solid adenocarcinomas, any nuclear TTF-1 expression was present in 69% of tumors.23 The subgroup with the lowest rate (50% to 60%) is mucinous adenocarcinoma (see subsequent Chapter 76). TTF-1 positivity is most helpful in differential diagnosis of tumors with a solid growth pattern (Fig. 75.12).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree