Chapter 48 Acute Gastrointestinal Hemorrhage
Acute gastrointestinal (GI) hemorrhage is a common clinical problem with diverse manifestations. This bleeding may range from trivial to massive and can originate from almost any region of the GI tract, including the pancreas, liver, and biliary tree. Although no demographic group is spared, the annual incidence of approximately 170 cases/100,000 adults increases steadily with advancing age, and is slightly more common in men than women.1 Furthermore, GI hemorrhage accounts for 1% to 2% of acute admissions, resulting in over 300,000 annual hospitalizations in the United States.2 It is also a common complication in patients hospitalized for other illnesses, especially surgical patients. Although the total economic burden of GI hemorrhage has not been formally assessed, annual estimates have suggested that diverticular bleeding alone costs the health care system in excess of 1.3 billion dollars.3
Management of these patients is frequently multidisciplinary, involving emergency medicine, gastroenterology, intensive care, surgery, and interventional radiology. The importance of early surgical consultation in the care of these patients cannot be overemphasized.4 In addition to aiding in the resuscitation of the unstable patient, in some settings the surgical endoscopist establishes the diagnosis and initiates therapy. Even when the gastroenterologist assumes this role, early collaboration with the surgeon permits the establishment of goals and limits for initial nonoperative therapy. Ultimately, 5% to 10% of patients hospitalized for bleeding require an operation intervention. Prompt surgical consultation permits more time for preoperative preparation and evaluation, as well as patient and family education, if urgent surgical intervention become necessary.1
Most patients with an acute GI hemorrhage stop bleeding spontaneously. This allows time for a more elective evaluation. However, in almost 15% of cases, major bleeding persists, requiring emergent resuscitation, evaluation, and treatment.5 Improvements in the management of such patients, primarily by means of early endoscopy and directed therapy, have significantly reduced the length of hospitalization. Despite this, mortality remains more than 5% and is significantly higher in those initially hospitalized for other reasons. This discrepancy between therapeutic advances and outcomes is probably related to the aging of the population, with an increase in comorbidity. Today, the patient requiring operative intervention is both older and sicker than in the past.
Hemorrhage can originate from any region of the GI tract and is typically classified based on its location relative to the ligament of Treitz. Upper GI hemorrhage from proximal to the ligament of Treitz accounts for more than 80% of cases of acute bleeding.1 Peptic ulcer disease and variceal hemorrhage are the most common causes. Most lower GI bleeding is from the colon, with diverticula and angiodysplasias accounting for most cases. In less than 5% of patients, the small intestine in responsible.1 Obscure bleeding is defined as hemorrhage that persists or recurs after negative endoscopy. Occult bleeding is not apparent to patients until they present with symptoms related to the anemia. Determination of the site of bleeding is important for directing diagnostic interventions with minimal delay. However, attempts to localize the source should never precede appropriate resuscitative measures.
Approach to the Patient
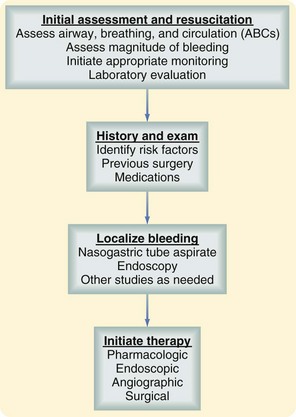
In patients with GI bleeding, several fundamental principles of initial evaluation and management must be followed. A well-defined and logical approach to the patient with GI hemorrhage is outlined in Figure 48-1. On presentation, a rapid initial assessment permits determination of the urgency of the situation. Resuscitation is initiated with stabilization of the patient’s hemodynamic status and the establishment of a means for monitoring ongoing blood loss. A careful history and physical examination should provide clues to the cause and source of the bleeding and identify any complicating conditions or medications. Specific investigation should proceed to refine the diagnosis. Therapeutic measures are then initiated, bleeding is controlled, and recurrent hemorrhage is prevented.
Risk Stratification
Not all patients with GI bleeding require hospital admission or emergent evaluation. For example, the patient with a small amount of rectal bleeding that has ceased can generally be evaluated on an outpatient basis. Clearly, in many patients, the decision making is less straightforward. Others require admission and observation but may be further evaluated with endoscopy on a more selective basis. Several prognostic factors have been associated with adverse outcomes including the need for emergent operation and death (Box 48-1).6 These factors should be considered during the initial assessment and resuscitation of patient with GI hemorrhage. For example, patients older than 60 years have higher mortality rates than their younger counterparts and should be evaluated more cautiously. This increased morbidity may be a reflection of concomitant disease. The deleterious effects of cardiac, renal, pulmonary, and hepatic comorbidity should all be considered when evaluating patient with GI bleeding. For example, one study has estimated that bleeding patients with significant renal disease have a mortality of almost 30%, which this increases to 65% in the presence of acute renal failure.7 Other factors, including the magnitude of the initial hemorrhage, persistence or recurrence of the bleeding, and onset of bleeding during hospitalization for another illness also contributes to increased morbidity and mortality.
Box 48-1 Risk Factors for Morbidity and Mortality in Acute Gastrointestinal Hemorrhage
Systolic blood pressure <100 mm Hg on presentation
Persistent or recurrent hemorrhage
Onset of hemorrhage during hospitalization
Considerable effort has been devoted to the development of risk-scoring tools to facilitate patient triage. These scoring systems have been used to predict the risk of rebleeding and mortality, evaluate the need for intensive care unit admission (ICU), and determine the need for urgent endoscopy. Some scoring systems are nonspecific to GI bleeding (e.g., APACHE II scores) but can provide general information about the patient’s condition and risk of adverse outcomes. There have also been attempts to develop disease specific scoring systems, such as the BLEED classification, that uses five criteria8: ongoing bleeding, systolic blood pressure less than 100 mm Hg, prothrombin time more than 1.2 times control, altered mental status, and unstable comorbid disease process that would require ICU admission. If any one of these criteria is present, the model predicts an approximately threefold increase in the risk of recurrent hemorrhage, need for surgical intervention, or death. Other systems take into account endoscopic findings that can improve on their predictive accuracy. Such scoring systems have been almost exclusively used in research studies, however, and until these have been prospectively validated for everyday clinical practice, they should only be applied in the context of clinical judgment.
History and Physical Examination
The medical history may provide clues to the diagnosis. Chronic blood loss may lead to non-GI end-organ symptoms, such as syncope, angina, and even myocardial infarction. Antecedent vomiting may suggest a Mallory-Weiss tear, whereas weight loss raises the specter of malignancy. Even demographic data may prove useful—older patients bleed from lesions such as angiodysplasias, diverticula, ischemic colitis, and cancer, whereas younger patients bleed from peptic ulcers, varices, and Meckel’s diverticula. A past history of GI disease, bleeding, or operation should immediately begin to focus the differential diagnosis. Antecedent epigastric distress may point to a peptic ulcer, whereas previous aortic surgery suggests the possibility of aortoenteric fistula. A history of liver disease prompts a consideration of variceal bleeding. Medication use may also be revealing. A history of ingestion of salicylates, nonsteroidal anti-inflammatory drugs (NSAIDs), and/or selective serotonin-reuptake inhibitors (SSRIs) is common, particularly in older patients.9 These medications are associated with GI mucosal erosions typically seen in the upper GI tract, but occasionally in the small bowel and colon. GI bleeding in the setting of anticoagulation therapy, warfarin or low-molecular-weight heparin, is still usually the result of GI pathology and should not be ascribed to the anticoagulation alone.10
Localization
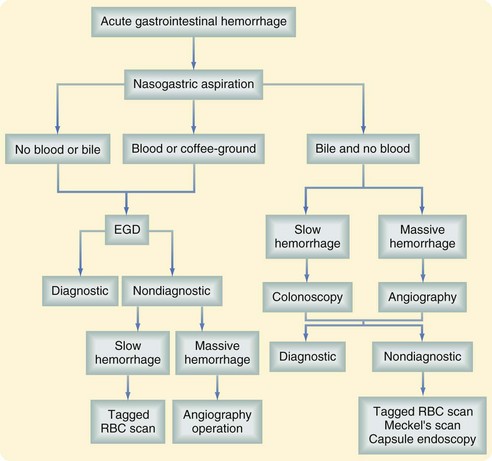
Subsequent management of the patient with acute GI hemorrhage depends on localization of the site of the bleeding. An algorithm for the diagnosis of acute GI hemorrhage is shown in Figure 48-2.
Although melena is usually from the upper GI tract, it can be the result of bleeding from the small bowel and/or colon. Similarly, hematochezia is sometimes the consequence of brisk upper GI bleeding. One approach to distinguishing these possibilities is the insertion of a nasogastric (NG) tube and examination of the aspirate. Although hematemesis is usually diagnostic of an upper GI bleed, the NG tube is still useful to assess the rate of ongoing bleeding and to begin to remove blood from the stomach to permit endoscopy. If the aspirate is positive, this effectively localizes the lesion. The presence of red blood or a coffee grounds appearance suggests an upper GI source. Testing for occult blood is rarely necessary. The return of bile from a gastric aspirate suggests that the duodenum has been sampled. Although a bilious, nonbloody, gastric aspirate generally excludes the upper GI tract, these findings can occasionally be misleading. One study found that only 6 of 10 yellow-green nasogastric aspirates actually tested positive for bile.11 Similarly, almost 20% of patients with a clear aspirate are still bleeding from an upper GI source.2 In patients with melena or even hematochezia from an upper lesion, the NG aspirate may be negative in the presence of significant duodenal bleeding and a competent pylorus preventing duodenogastric reflux. These considerations suggest that although the findings of the NG aspirate could be helpful, almost all patients with significant bleeding should undergo upper endoscopy.
Upper endoscopy under these circumstances is highly accurate for identifying an upper GI lesion and, if negative, for directing attention to a lower GI source. To maximize efficacy, early endoscopy should be performed within 24 hours, even in stable patients.12 Early endoscopy with directed therapy has been shown to reduce resource use and transfusion requirements, and shorten hospital stay. The exact definition and timeline of an early endoscopy has been well studied and refined. Although there is little argument that in an unstable patient, an urgent endoscopy is often required, in those with overt sign of bleeding but who are stable, an endoscopy within 6 or 12 hours has not been shown to be of any additional benefit to endoscopies performed within 24 hours.13,14
Subsequent evaluation depends on the results of the upper endoscopy and volume of the bleeding (Fig. 48-2). Angiography or even surgery may prove necessary for massive hemorrhage, precluding endoscopy, from the upper or lower GI tract. For slow or intermittent bleeding from the lower GI tract, colonoscopy is now the initial diagnostic maneuver of choice. When this is nondiagnostic, the tagged red blood cell (RBC) scan is usually used. For obscure bleeding, usually from the small bowel, capsule endoscopy has become the appropriate study. These diagnostic procedures are discussed subsequently in greater detail.
Treatment
For most patients, bleeding has ceased and therapeutic options are applied to prevent recurrence. The risk of recurrent bleeding, and therefore the need for preventative intervention, depends on the characteristics of the lesion, magnitude of the initial hemorrhage, and specific patient. For example, although the risk of recurrent diverticular hemorrhage is relatively low, elective colonic resection may still be appropriate for a patient with significant coronary disease who has already suffered a major bleed. For the approximately 15% of patients who continue to bleed, therapy is more urgent. In patients with hemodynamic instability, an appropriate goal is to institute therapy within 2 hours of presentation. This depends on the development of institution-specific protocols for the multidisciplinary management of these patients.2 The availability of an endoscopist trained in techniques of hemostasis and appropriate support staff is critical. Similarly, angiographic expertise must be immediately accessible. Despite the relatively new modalities available for nonoperative control of bleeding, early involvement of the surgical team remains essential.
Acute Upper GI Hemorrhage
Upper GI bleeding refers to bleeding that arises from the GI tract proximal to the ligament of Treitz; it accounts for almost 80% of significant GI hemorrhage. The causes of upper GI bleeding are best categorized as nonvariceal sources or bleeding related to portal hypertension (Table 48-1). The nonvariceal causes account for approximately 80% of this bleeding, with peptic ulcer disease being the most common.1 In the remaining 20% of patients, most of whom have cirrhosis, portal hypertension can lead to the development of gastroesophageal varices, isolated gastric varices, or hypertensive portal gastropathy, any of which can be the source of an acute upper GI bleed. Although patients with cirrhosis are at high risk of developing variceal bleeding, nonvariceal sources account for most upper GI bleeds, even in these patients.2 However, because of greater morbidity and mortality of variceal bleeding, patients with cirrhosis should generally be assumed to have variceal bleeding; appropriate therapy should be initiated until an emergent endoscopy has demonstrated another cause for the hemorrhage.
Table 48-1 Common Causes of Upper Gastrointestinal Hemorrhage*
| NONVARICEAL BLEEDING* | PORTAL HYPERTENSIVE BLEEDING† |
|---|---|
| 30%-50% Peptic ulcer disease | Gastroesophageal varices >90 |
| 15%-20% Mallory-Weiss tears | Hypertensive portal gastropathy, <5 |
| 10%-15% Gastritis or duodenitis | Isolated gastric varices, rare |
| 5%-10% Esophagitis | |
| 5% Arteriovenous malformations | |
| 2% Tumors | |
| 5% Others |
Adapted from Ferguson CB, Mitchell RM: Nonvariceal upper gastrointestinal bleeding: Standard and new treatment. Gastroenterol Clin North Am 34:607–621, 2005.
The foundation for the diagnosis and management of patients with an upper GI bleed is an upper endoscopy. A number of studies have demonstrated that early EGD, within 24 hours, results in reductions in blood transfusion requirements, decrease in the need for surgery, and shorter length of hospital stay. Endoscopic identification of the source of bleeding also permits an estimate of the risk of subsequent or persistent hemorrhage and facilitates operative planning, should that prove necessary. In general, 20% to 35% of patients undergoing upper GI endoscopy will require a therapeutic endoscopic intervention, and 5% to 10% will eventually require surgery.12
As noted, it is somewhat surprising that studies have not shown any benefits in performing an endoscopy sooner (within 6 or 12 hours) than within 24 hours.13,14 Although the best tool for localization of the bleeding source is an EGD, this intervention is associated with increased risk and poor visualization in the acute setting, which may offset some of its benefits. In 1% to 2% of patients with upper GI hemorrhage, the source cannot be identified because of the excessive blood impairing the visualization of the mucosal surface.15 Aggressive lavage of the stomach with room temperature normal saline solution prior to the procedure can be helpful. Evidence has suggested that a single bolus injection of IV erythromycin, which stimulates gastric emptying, can significantly improve visualization.16 If identification of the source is still not possible, angiography may be appropriate in the reasonably stable patient, although operative intervention should be seriously considered if the blood loss is extreme or the patient hemodynamically unstable. A tagged RBC scan is seldom necessary with a confirmed upper GI bleed and contrast studies are usually contraindicated because they will interfere with subsequent maneuvers.
Specific Causes of Upper Gastrointestinal Hemorrhage
Nonvariceal Bleeding
Peptic Ulcer Disease
Peptic ulcer disease (PUD) still represents the most frequent cause of upper GI hemorrhage, accounting for approximately 40% of all cases.2 Approximately 10% to 15% of patients with PUD develop bleeding at some point in the course of their disease. Bleeding is the most frequent indication for operation and the principal cause for death in PUD. PUD is discussed in more detail in Chapter 49; this discussion focuses only on bleeding from ulcer disease.
The epidemiology of peptic ulcer continues to change. The incidence of uncomplicated peptic ulcer disease has declined dramatically. This change has been attributed to better medical therapy, including the use of proton pump inhibitors (PPIs) and regimens for the eradication of Helicobacter pylori (H. pylori). Despite this overall decline in ulcer frequency, the number of patients undergoing operation for ulcer-related complications had remained surprisingly stable until now. Recent reports, however, are documenting a decline in the rate of some, but not all, ulcer-related complications requiring surgical intervention. Although the need for surgery for perforated PUD has declined, the rate of bleeding PUD requiring surgical intervention has remained stable.17 Some population-based studies in older patients have documented an increase in PUD bleeding requiring hospital admission.18 Thus, when surgeries for upper GI hemorrhage are undertaken, they are now typically performed in the oldest and often the sickest patients.
Treatment
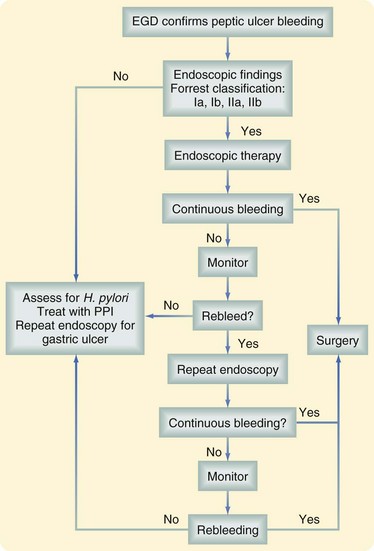
Figure 48-3 outlines an approach to management. As noted, patients with clinical evidence of a GI bleed should receive an endoscopy within 24 hours and, while awaiting this procedure, they should be treated with a PPI. Although this approach has been shown to reduce the stigmata of a recent hemorrhage at index endoscopy, it has had no impact on clinical outcomes, such as transfusion requirement, mortality, or need for surgery. Nevertheless, it is believed to be a cost-effective intervention for those suspected of having an upper GI bleed.19
After the index endoscopy, treatment strategies depend on the appearance of the lesion at endoscopy. Endoscopic therapy is instituted if bleeding is active or, when bleeding has already stopped, if there is a significant risk of rebleeding. The ability to predict the risk of rebleeding permits prophylactic therapy, closer monitoring, and earlier detection of hemorrhage in high-risk patients. The Forrest classification was developed in an attempt to assess this risk based on endoscopic findings and stratify the patients into low-, intermediate-, and high-risk groups (Table 48-2). Endoscopic therapy is recommended in cases of active bleeding as well as those with a visible vessel (Forrest I to IIa). In case of an adherent clot (Forrest IIb), the clot is removed and the underlying lesion evaluated. Ulcers with a clean base or black spot, secondary to hematin deposition, are generally not treated endoscopically.
Table 48-2 Forrest Classification for Endoscopic Findings and Rebleeding Risks in Peptic Ulcer Disease
| Classification | ||
|---|---|---|
| GRADE | DESCRIPTION | REBLEEDING RISK |
| Ia | Active, pulsatile bleeding | High |
| Ib | Active, nonpulsatile bleeding | High |
| IIa | Nonbleeding visible vessel | High |
| IIb | Adherent clot | Intermediate |
| IIc | Ulcer with black spot | Low |
| III | Clean, nonbleeding ulcer bed | Low |
Medical Management
In cases of a confirmed peptic ulcer bleed, PPIs have been shown to reduce the risk of rebleeding and the need for surgical intervention. Therefore, patients with a suspected or confirmed bleeding ulcer should be started on a PPI.20 Unlike perforated ulcers, which are generally associated with H. pylori infection, the association between H. pylori infection and bleeding is weaker. Only 60% to 70% of patients with a bleeding ulcer are H. pylor–positive.21 This has generated some discussion about the importance of H. pylori treatment in patients with a bleeding peptic ulcer. Several studies and a large meta-analysis, however, have shown that H. pylori treatment and eradication, in patients who test positive for the infection, result in decreased rebleeding.22 Importantly, once the H. pylori infection has been eradicated, there is no need for long-term acid suppression and no increased risk of further bleeding with this approach.23
In patients who are taking ulcerogenic medications such as NSAIDs or selective serotonin reuptake inhibitors (SSRIs), and present with a bleeding GI lesion, these agents should be stopped. Patients should be started on a nonulcerogenic alternative, if possible. In those taking NSAIDs, more specific cycloxygenase-2 (COX-2) inhibitors, which are associated with reduced ulceration, had been a promising alternative. Concerns regarding the cardiotoxicity of these drugs have resulted in their withdrawal from the market, reducing the clinical use of these alternative medicines. Further affecting the popularity of these medications have been population-based studies showing that not all COX-2 inhibitors result in a decreased incidence of upper GI complications.24 Therefore, an alternative approach has been to identify ways to reduce the adverse GI side effects of NSAIDs. To this end, studies have shown that H. pylori eradications in infected patients who are about to start these medications can reduce the incidence of adverse GI side effects, including bleeding.25 These studies highlight the synergistic effect of H. pylori and NSAIDs. Although this approach can have a preventive role in regard to GI bleeding, NSAIDs cannot be recommended for those who present with a bleed, even after H. pylori eradication.26
Endoscopic Management
Once the bleeding ulcer has been identified, effective local therapy can be delivered endoscopically to control the hemorrhage. Available endoscopic options include epinephrine injection, heater probes, and coagulation, as well as the application of clips. Epinephrine injection (1 : 10,000) to all four quadrants of the lesion is successful in controlling the hemorrhage. It has been shown that large-volume injection (>13 mL) is associated with better hemostasis, suggesting that the endoscopic injection works in part by compressing the bleeding vessel and inducing tamponade.27 Epinephrine injection alone is associated with a high rebleeding rate; standard practice is to provide combination therapy. This usually means the addition of thermal therapy to the injection. The sources of thermal energy can be heater probes, monopolar or bipolar electrocoagulation, or laser or argon plasma coagulation (APC). The most commonly used energy sources are electrocoagulation for bleeding ulcers and APC for superficial lesions. A combination of injection with thermal therapy achieves hemostasis in 90% of bleeding PUDs. The role of hemoclips is less clear; several studies have reported mixed results. Hemoclips (Fig. 48-4), which can be difficult to apply, may be particularly effective when dealing with a spurting vessel because they provide immediate control of hemorrhage.
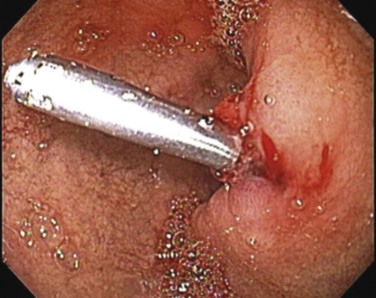
FIGURE 48-4 Hemoclip that has been applied to a bleeding duodenal ulcer.
(Courtesy Dr. Linda S. Lee, Brigham and Women’s Hospital, Boston.)
Rebleeding of an ulcer is associated with a significant increase in mortality, and careful observation of patients at high risk of rebleeding, using criteria previously described, is important. In those who rebleed, the role of a second attempt at endoscopic control has been controversial but has been validated. For example, one study has demonstrated that a second attempt at endoscopic hemostasis is successful in 75% of patients.28 Although this will fail in 25% of patients, who will then require emergent surgery, there does not appear to be any increase in morbidity or mortality with this treatment approach. Therefore, most clinicians would now encourage a second attempt at endoscopic control before subjecting the patient to surgery.
Surgical Management
Despite significant advances in endoscopic therapy, approximately 10% of patients with bleeding ulcers still require surgical intervention for effective hemostasis. Identifying patients who are likely to fail endoscopic therapy is difficult, however, and timing of surgery has been greatly debated. To assist in this decision making, several clinical and endoscopic parameters have been proposed that are thought to identify patients at high risk of failed endoscopic therapy. The clinical factors to consider are shock and a low hemoglobin level at presentation. At the time of endoscopy, although the Forrest classification is the most important indicator of rebleeding risk, the location and size of the ulcer are also significant. Ulcers larger than 2 cm, posterior duodenal ulcers, and gastric ulcers have a significantly higher risk of rebleeding.29,30 Patients with these characteristics need closer monitoring and possibly earlier surgical intervention. Clearly, clinical judgment and local expertise must play a critical role in this decision.
Indications for surgery were traditionally based on the blood transfusion requirements. Increased blood transfusions are clearly associated with increased mortality. Although a less definitive criterion than it was formerly, most surgeons still consider an ongoing blood transfusion requirement in excess of 6 U an indication for surgical intervention, particularly in older patients, although an 8- to10-U loss may be more acceptable for younger patients. Current indications for surgery for peptic ulcer hemorrhage are summarized in Box 48-2. Secondary or relative indications include a rare blood type or difficult crossmatch, refusal of transfusion, shock on presentation, advanced age, severe comorbid disease, and a bleeding chronic gastric ulcer for which malignancy is a concern.
Box 48-2 Indications for Surgery in Gastrointestinal Hemorrhage
Hemodynamic instability despite vigorous resuscitation (>6 U transfusion)
Failure of endoscopic techniques to arrest hemorrhage
Shock associated with recurrent hemorrhage
Continued slow bleeding with a transfusion requirement >3 U/day
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree