Chapter 50
Acute Deep Venous Thrombosis
Clinical and Diagnostic Evaluation
Fedor Lurie, David Paolini
Based on a chapter in the seventh edition by Joann Lohr and Nathan M. Griffith
Acute thrombosis of the deep veins of the lower extremity remains one of the most significant public health problems. It affects more than 350,000 people in the United States annually and is responsible for between 100,000 and 300,000 deaths.1,2 Its economic impact is substantial; in the United States alone, the annual cost of deep venous thrombosis (DVT) is estimated to be 7.5 to 39 billion in 2010 U.S. dollars.3 Although it is generally accepted that early diagnosis of acute DVT is necessary for achieving the best outcomes, detection of this disease is challenging as its early stages are frequently subclinical. To address this challenge, strategies have been developed that include risk assessment as the first diagnostic step. Adaptation of these strategies and investigation of their effectiveness have demonstrated that structured clinical diagnosis of acute DVT is more accurate than was previously thought.
New therapeutics extend the ability to safely treat acute thrombotic episodes, to prevent rethrombosis, and to prevent DVT in high-risk patients. The result is a continuous shift toward early treatment of the majority of the patients based on the clinical information, reserving more expensive diagnostic tests for patients who require invasive treatment.4
Clinical Assessment
The clinical manifestations of acute DVT vary from an absence of symptoms and signs to the dramatic presentation of phlegmasia cerulea dolens and venous gangrene. Such broad variation is a result of multiple pathologic processes, with different timing and severity occurring during an acute thrombotic episode. Anatomic distribution of thrombosed venous segments, degree of occlusion in each affected vein, time of thrombus development, severity of inflammation, functional status of the lymphatic system, preexisting venous and lymphatic insufficiency, and many other factors determine the presentation at the time of clinical evaluation. Symptoms tend to be more severe in proximal DVT and when more venous segments are involved and less severe in calf vein thrombosis and in older and postoperative patients. The most common symptoms and signs of DVT are dull ache or pain in the leg, tenderness, swelling, erythema, cyanosis, and fever. Edema, cyanosis, and pain are features of phlegmasia cerulea dolens. Venous gangrene is a rare condition that occurs usually in patients with cancer, can occur with heparin-induced thrombocytopenia with thrombosis, and is generally associated with warfarin-mediated protein C depletion.5,6
Diagnostic properties of signs and symptoms of DVT and their combinations have been extensively studied.7–11 The most common symptom of calf pain has been reported to have sensitivity between 75% and 91% and specificity between 3% and 87%. The reported sensitivity of calf swelling for DVT diagnosis ranges from 35% to 97%, and its reported specificity ranges from 8% to 88%. In part, such variability is due to the high prevalence of the same signs and symptoms in patients without DVT.11 In patients at high risk for DVT, the diagnostic value of clinical signs and symptoms is substantially higher. For example, in cancer patients, the negative predictive value of absence of swelling is as high as 97% for outpatients and 92% for inpatients.12 A valid alternative diagnosis, such as cellulitis or a musculoskeletal disorder, that explains the presence of signs and symptoms may also improve the negative predictive value of the clinical examination.13
High variability and lack of specificity limit the role of clinical examination in patients with suspected DVT. A meta-analysis of studies evaluating individual clinical features of DVT showed that only a past history of DVT and malignant disease are useful for diagnosis, and no individual clinical feature is useful for ruling out DVT.14 The delay of clinical manifestations makes the clinical diagnosis of DVT even more challenging. On average, patients are seen by a medical professional 4.4 days after onset of symptoms, and in more than 22% of patients, the onset of symptoms occurred more than 10 days before presentation.15,16 For these reasons, withholding treatment on the basis of clinical evaluation in primary care settings leads to inadequate management of more than 10% of patients with DVT.17
Diagnostic Tests
D-Dimer
The concentration of D-dimer in plasma has become a widely used marker for the diagnosis of DVT. D-dimer is a product of fibrin proteolysis by plasmin; therefore, its elevated levels signify that fibrinolysis of complexed fibrin is taking place. This commonly occurs as part of the response to injury in patients undergoing surgical procedures or trauma, as part of the normal physiologic changes during pregnancy, and as part of the pathophysiologic process in patients with cancer or thrombotic disorders. The degree of D-dimer elevation in patients with DVT varies with the size and extent of the thrombus, the time from its onset, and the use of anticoagulation. Diagnostic properties of D-dimer vary substantially, depending on the assay used for its detection. Currently used assays differ in the specificity of antibody to various binding sites on the D-dimer molecule, in units of measurements, and in reference values. Their sensitivity for DVT diagnosis ranges from 60% to 96%.18,19 Assays also vary with respect to speed of testing, cost, and labor involved. The most sensitive of the assays, conventional enzyme-linked immunosorbent assay, is also the most laborious and slow.18,20–22 Rapid point-of-care assays are probably the most practical as the results are obtainable within minutes and their sensitivity is comparable to that of the enzyme-linked immunosorbent assay.23,24
Low specificity limits the use of D-dimer in patients with suspected DVT. However, although concentration of D-dimer below the cutoff value indicates a very low probability of DVT, it does not exclude it with sufficient accuracy, especially in cases of distal thrombi, use of anticoagulation, or long duration between the onset of thrombosis and testing.19,22,25 Similar to clinical evaluation, the use of D-dimer as a single diagnostic tool may result in inadequate management of more than 15% of patients with suspected DVT.19 D-dimer is best integrated with a clinical risk assessment of the patient, as discussed later in this chapter.
Duplex Ultrasonography
Duplex ultrasonography remains the dominant diagnostic test of choice for the detection of DVT. It has almost completely replaced venography because of its accuracy, lack of radiation, portability, noninvasiveness, and relative cost-effectiveness. In addition, ultrasound has the ability to distinguish among nonvascular pathologic processes, such as inguinal adenopathy, Baker’s cyst, abscess, and hematoma.
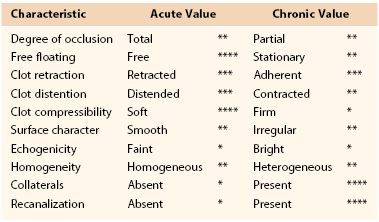
The technique of venous duplex scanning is discussed in Chapter 18. Duplex ultrasound diagnostic criteria for DVT include increased intraluminal echogenicity, increased venous diameter, inability of the vein to collapse under a moderate pressure from the transducer, absence of spontaneous blood flow, and absence of flow augmentation with distal compression (Table 50-1; Figs. 50-1 to 50-5). Among these factors, inability to compress the vein is the most widely used objective criterion for the diagnosis of DVT.26 Even as a stand-alone criterion, noncompressibility has excellent sensitivity and specificity for the detection of proximal DVT, with sensitivity and specificity of 97% and 94%, respectively, for proximal DVT.27
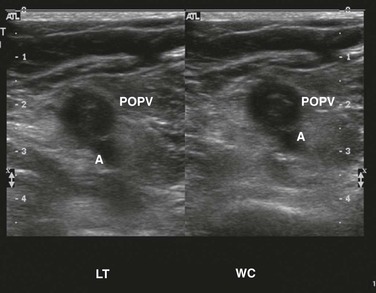
Figure 50-1 Totally occluding, acute popliteal vein deep venous thrombosis without (left) and with (right) compression. A, Artery; LT, left; POPV, popliteal vein; WC, with compression.
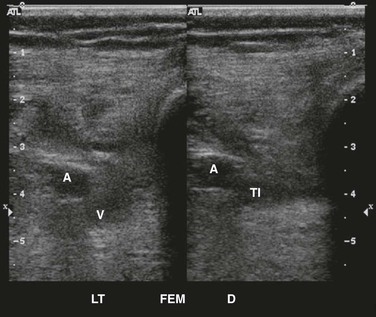
Figure 50-2 Totally occluding, age-indeterminate deep venous thrombosis in the left femoral vein without (left) and with (right) compression. A, Artery; D, distal; FEM, femoral; LT, left; TI, total indeterminate; V, vein.
Figure 50-3 Partially occluding, chronic deep venous thrombosis in the left common femoral and deep femoral veins without (left) and with (right) compression. A, Artery; DFV, deep femoral vein; FV, femoral vein; PC, partially occluding chronic; W/C, with compression.
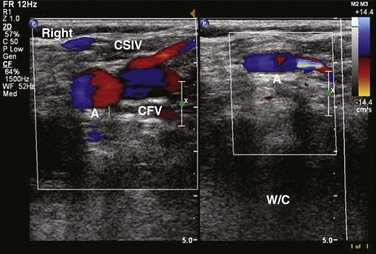
Figure 50-4 Longitudinal image of the confluence of the superficial inguinal vein (CSIV) and common femoral vein (CFV) without (left) and with (right) compression (W/C). A, Artery.
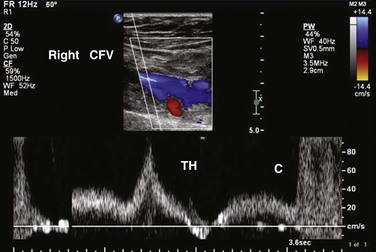
Figure 50-5 Right common femoral vein (CFV) Doppler ultrasound image showing thigh augmentation (TH) and calf augmentation (C).
A significant limitation of compression ultrasound is its lack of accuracy in the evaluation of calf veins. Addition of evaluation of venous flow with color Doppler and spectral Doppler can improve the accuracy of compression ultrasonography.
Because the risk of proximal extension of the thrombus exists in patients with DVT even when they receive appropriate anticoagulation,28 repeated ultrasonography has been advocated.29 Additional ultrasound 7 to 14 days after an initial examination can reduce the rate of thromboembolic complications to 1% during a 3-month follow-up period30 compared with 2.5% with no additional testing.31,32 The diagnostic gain from repeated ultrasound is estimated to be 1.34% of positive studies, with a positive predictive value of 89%,14 which makes this option cost-ineffective.33 Limiting indications for repeated ultrasonography to patients with positive D-dimer assays and normal initial ultrasound findings increases its clinical utility.13,30,34
Pitfalls in venous duplex imaging include misidentification of veins; missing of duplicate venous systems; systemic illness or hypovolemia resulting in decreased venous distention; suboptimal imaging in obese or edematous patients; and areas not amenable to compression, such as the iliac veins, the femoral vein at the adductor canal, and the subclavian veins. As with most ultrasound-based imaging studies, the quality of the examination depends on the skill of the technologist performing the study.
Despite its utility, duplex ultrasound has limitations. A meta-analysis showed that the sensitivities of compression ultrasound for detection of proximal and distal DVT are 94.2% and 63.5%, respectively, resulting in an overall sensitivity of 89.7% and specificity of 93.8%.14 The diagnostic properties of duplex ultrasound are substantially different in the subpopulation of patients with suspected DVT. For example, in patients with a high Wells score, sensitivity is 91% with specificity of 100%, whereas sensitivity and specificity are 61% and 99%, respectively, in patients with an intermediate Wells score and 67% and 98% in patients with a low Wells score.35 In asymptomatic patients, sensitivities of duplex ultrasound for detection of proximal and distal DVT are 66.7% and 39.0%, respectively, with an overall specificity of 96.5%.14 These data indicate that the use of duplex ultrasound in low-risk patients may result in a significant number of false positives. In symptomatic patients, duplex ultrasound can reliably rule out proximal but not distal DVT, and in asymptomatic patients, ruling out DVT on the basis of sonographic findings is highly questionable.
Plethysmography
Strain-gauge techniques and air plethysmography have been almost entirely replaced by duplex ultrasound in the diagnosis of DVT but still deserve mention. These techniques allow assessment of the impact of thrombus on venous outflow from the lower extremity and make it possible to quantitatively assess thrombus resolution and recanalization. Their diagnostic properties, however, are inferior to duplex ultrasound, with specificities from 84% to 93% and sensitivities between 69% and 89%.36
Plethysmographic techniques for evaluation of venous obstruction involve thigh compression with a pneumatic cuff inflated to the pressure above the existing venous pressure, thereby filling the entire capacity of the leg’s veins. The central venous pressure at this point is assumed to be close to zero and the pressure in the veins of the legs equal to the pressure in the cuff. When the cuff is rapidly released, change in the leg volume reflects flow rate in the veins. Together with the known pressure gradient, this rate allows calculation of the resistance to venous outflow. The position of the occlusive cuff on the leg determines which venous segments are included in the measurements, and the position of the leg influences the outflow rate. These two sources of variability in the measurements may result in even lower sensitivity than is generally reported.37 Because the occlusive cuff is placed on the thigh, plethysmographic diagnosis of calf DVT is especially problematic. Regardless of its limitations, plethysmography remains the only available tool for physiologic monitoring of thrombus resolution by evaluation of venous outflow, which is an indirect measure of venous obstruction. Because venous outflow measurements can be affected by the degree of collateral drainage, this technique is insensitive as a measure of thrombus resolution of the target deep veins.
Venography
Contrast venography for the sole purpose of diagnosing DVT is largely of historical interest; however, it is invaluable when it is used as part of the treatment plan. Reasons for its dramatic decline are obvious in light of the utility, accuracy, and safety of duplex ultrasonography. It is expensive and inconvenient compared with other diagnostic modalities and potentially causes patient discomfort.38,39 Complications of the examination include nephrotoxicity, allergy, phlebitis, and the need for intravenous access. Nevertheless, contrast venography can be useful when other studies have not produced a solid diagnosis, making it important to retain this technology for diagnosis and therapeutics.
There are two basic techniques for the performance of contrast venography. The Rabinov-Paulin technique uses spot film, and the long-leg technique uses cine film. Although both techniques are useful, Lensing et al40 demonstrated that 20% of examinations were inadequately interpreted by the Rabinov-Paulin technique versus only 2% by the long-leg technique. In addition, they went on to show a 21% interobserver disagreement with the Rabinov-Paulin technique, whereas the long-leg technique had only a 4% disagreement.
Despite the disadvantages of contrast venography, it continues to be perceived as the “gold standard” for the diagnosis of acute DVT. Comparisons of contrast venography with duplex ultrasound using D-dimer for the confirmation of thrombus with both diagnostic modalities confirmed a sensitivity and specificity of 96% and 91%, respectively, for contrast venography and 78% and 97% for duplex ultrasonography.41 Moreover, in the same study, calf vein acute DVT was diagnosed in only 74% of patients already positively diagnosed with contrast venography. Similarly, Ozbudak et al42 showed that contrast venography diagnosed 19% of acute DVT that duplex ultrasound failed to identify. de Valois et al43 demonstrated that duplex ultrasonography accomplished a 92% sensitivity and 90% specificity compared with contrast venography, concluding that contrast venography should be used as the “golden backup” when the diagnosis of acute DVT remains in question after a venous duplex examination.
Computed Tomographic Venography
Computed tomographic arteriography is an excellent technique for the diagnosis of pulmonary embolism. However, its venous counterpart, computed tomographic venography (CTV), has yet to gain traction for the diagnosis of acute DVT in the lower or upper extremities. The diagnostic capabilities of CTV are remarkable in the thigh and pelvis compared with duplex ultrasound; it has a sensitivity of 98% and specificity of 100% in the thigh and sensitivity and specificity of 94% and 100%, respectively, in the pelvis. Overall positive predictive and negative predictive values are 92% and 100%, respectively.44–47 Thomas et al48 conducted a meta-analysis of 13 studies evaluating CTV for the diagnosis of DVT in patients with suspected DVT and pulmonary embolism (PE). The sensitivity ranged from 71% to 100% and the specificity from 93% to 100%. The pooled estimate of sensitivity was 95.5%, whereas the pooled estimate of specificity was 95.2%. They concluded that CTV has a sensitivity and specificity similar to those of ultrasonography for the diagnosis of acute DVT but must be viewed with caution, as duplex ultrasound does not have perfect sensitivity or specificity, which may lead to overestimation of the accuracy of CTV. In addition, when CTV is used in conjunction for evaluation of PE, it adds only 3 to 5 minutes to the examination, making it an attractive option as the sole diagnostic modality for acute lower extremity DVT.49
Not all authors have shown as promising results for diagnosis of acute DVT with CTV. Peterson et al50 demonstrated that although the sensitivity is high at 93%, producing a 97% negative predictive value, the ability of CTV to accurately diagnose DVT has a specificity of 71%, giving a positive predictive value of only 53%. Others have shown a 50% false-positive rate for CTV for pelvic DVT; at the same time, magnetic resonance venography had a 100% rate of false positivity.51 Moreover, CTV has not been well studied for acute calf vein DVT, making its role questionable as a comprehensive diagnostic modality for the lower extremity. Results of accuracy vary widely for CTV, probably owing to a pretest bias toward DVT as most patients are already being studied for PE simultaneously. Other disadvantages to CTV are that it is less cost-effective than duplex ultrasound, it involves the use of contrast material, and it uses radiation. The technique of computed tomography is described in Chapter 22.
Magnetic Resonance Venography
Magnetic resonance venography (MRV) has become an attractive option for vascular imaging. The technology is based on the detection of moving versus stationary tissues. MRV is less expensive than contrast venography but more expensive and less operator dependent than duplex ultrasound. In addition, MRV overcomes many of the anatomic limitations encountered with duplex ultrasound when the inferior vena cava and iliac vessels are imaged.
MRV can be used with or without contrast enhancement. Non–contrast-enhanced techniques include time-of-flight imaging and the phase-contrast technique relying on flow-related enhancement.52 Contrast-enhanced MRV can provide the user with three-dimensional imaging, provided contrast material is injected in a timed sequence. Postprocessing can then remove the arterial anatomy, leaving only the venous segments in the display image.53 Although it is not used to diagnose DVT, Figure 50-6 is a good example of time-resolved magnetic resonance imaging (MRI) of the central veins.
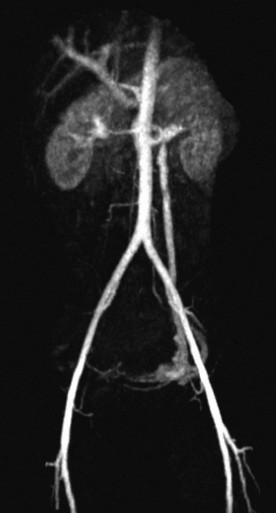
Figure 50-6 Time-resolved magnetic resonance imaging after intravenous administration of a long-acting contrast agent in a female patient with symptoms consistent with pelvic congestion syndrome. The image shows early reflux through the left ovarian vein into pelvic varices. The patient also has compression of the left renal vein between the superior mesenteric artery and aorta, consistent with the “nutcracker syndrome.”
When MRV is used to diagnose acute DVT, results are impressive for larger venous segments, but sensitivities diminish when smaller diameter veins are evaluated. Carpenter et al53 reported nearly identical findings compared with contrast venography in 97% of patients with acute DVT and 100% agreement in the extent of thrombus burden and whether thrombus was occlusive. Laissy et al54 found 100% sensitivity and specificity compared with contrast venography, with 95% sensitivity in detection of the extent of thrombus. These results were largely based on proximal vein segments in the abdomen, pelvis, and thigh. In fact, proximal disease confers a much higher sensitivity than tibioperoneal disease. There were 83% to 92% sensitivities for two congruent readers for acute calf DVT; the femoropopliteal venous segment had a 97% sensitivity for the same readers, whereas the iliofemoral segment had a 100% sensitivity.55 Moreover, vessel wall enhancement can be visualized with acute thrombus, allowing the examiner a crude detection of thrombus age.56
Comparisons between the two modalities of MRV have also been examined. Time-of-flight MRV and duplex examinations had sensitivities and specificities of 100% and 96%, respectively, compared with contrast venography. Positive and negative predictive values were 90% and 94%, respectively.53 When more proximal iliocaval DVT is examined, time-of-flight MRV had 100% sensitivity and specificity compared with contrast venography versus 87% and 85%, respectively, for duplex ultrasonography.54 Perhaps the most impressive and useful aspect of time-of-flight MRV was its 95% sensitivity and 99% specificity in detection of the proximal extent of thrombus in the iliocaval segment compared with 46% and 100%, respectively, for duplex ultrasonography.54 Contrast-enhanced MRV provides the examiner with 100% sensitivity and specificity for iliac thrombus and 100% sensitivity with a 97% specificity for the detection of femoral thrombus,57 while at the same time being more reliable in distinguishing the proximal extent of these thrombus burdens.56 With the addition of axial true fast imaging with steady-state precession, MRV has a sensitivity and specificity of 100% for iliocaval thrombus, 100% and 98% for femoral thrombus, and 68% and 94% for below-knee thrombus, producing an overall lower extremity sensitivity and specificity of 87% and 98%, respectively.57
Although MRV technology demonstrates excellent accuracy for the detection of acute DVT, several disadvantages can make the examination prohibitive. The examination demands a nonmoving patient and long imaging times that, when paired, can be a significant hurdle. The below-knee segments of venous anatomy are often paired, accounting for significant artifact during postprocessing of the images.52,58 In addition, gadolinium can be toxic in patients with renal dysfunction, and the need for frequent examinations can produce problematic utilization issues in larger institutions. However, MRV certainly has a role in the diagnosis of DVT, especially in the detection of thrombus in centrally located venous structures not always accessible to duplex ultrasonography. Not only is MRV useful for detection of hypogastric venous thrombosis,53 a remarkable 27% of patients without a detectable source of thrombus by duplex ultrasound who have sustained a PE had thrombus identified by MRV.59
More recently, Sampson et al60 conducted a meta-analysis regarding the accuracy of MRI for the diagnosis of acute DVT. The 14 reports included in the analysis showed a range of sensitivity from 0% to 100% with a specificity of 43% to 100% for the diagnosis of acute DVT. Their pooled estimate of sensitivity was 91.5%, and their pooled estimate of specificity was 94.8%.60 Here, again, the sensitivity and specificity were higher for proximal DVT than for below-knee thrombus. They concluded that MRI may offer an alternative for patients in whom ultrasound is inappropriate, is not feasible, or yields inconclusive results.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree