Chapter 51
Acute Deep Venous Thrombosis
Prevention and Medical Treatment
Juan I. Arcelus, Joseph A. Caprini
Venous thromboembolism (VTE) represents a serious medical problem that includes pulmonary embolism (PE) and deep venous thrombosis (DVT), most frequently of the legs, arms, or pelvis. Other less common sites for DVT include the renal veins, inferior or superior vena cava, or the right heart.
DVT of the legs may be divided into three types: namely, calf DVT involving the infrapopliteal venous system, proximal DVT involving the proximal leg veins up to the inguinal ligament, and iliofemoral DVT involving proximal DVT that extends above the inguinal ligament. This classification is critically important because the incidence of PE, postthrombotic syndrome (PTS), and treatment approach varies, as will be discussed later in this chapter. Thrombi from any of these locations can break off and result in PEs. Right atrial dilatation can also occur, which may open a dormant patent foramen ovale, allowing the thrombus to pass into the left atrium, and hence, into the systemic circulation (paradoxical embolus). Most often these thrombi travel to the brain, producing a so-called cryptogenic stroke, and rarely, the thrombi can embolize to the axillary artery or distally into the visceral arterial tree. It has been estimated that up to 25% of the normal population has a patent foramen ovale, and patent foramen ovales have been found in 50% of the stroke population >60 years of age.1 Physicians often do not think of stroke as a manifestation of DVT, and this represents a serious awareness problem in the opinion of the authors.
Epidemiology and Natural History of Venous Thromboembolism
VTE is a leading cause of death in the United States and kills 4 to 5 times more people annually than breast cancer or AIDS.2,3 The in-hospital mortality of VTE is 12%, which represents the number one preventable cause of deaths in hospitalized patients.4 The 1-year mortality for DVT and PE in elderly patients is 21% and 39%, respectively.5
A recent epidemiologic study suggests that in the United States alone, 613,423 nonfatal VTE events and 296,370 VTE-related deaths occur yearly.6 Approximately one-third of these VTE-related deaths occur in the community after hospital discharge, and one-third of all of these estimated fatal PE events present as sudden death. This analysis estimates that 200,000 nonfatal PEs occur annually, and among these patients, pulmonary hypertension is expected to develop in 4% of these survivors.7,8 This problem may result in reduced quality of life for affected individuals, representing another important complication of VTE not often recognized by the physician as a problem related to venous thrombosis. Patients with acute PE who eventually recover are estimated to have a 7-day case fatality rate of 34% if they experience a recurrent PE. This is dramatically different than the 7-day case fatality rate of 4% for recurrent DVT.9
The incidence of DVT has been estimated by Heit to be 117 of 100,000 person-years in the United States, and increases markedly with age and also in the presence of thrombophilia.9 Precipitating causes such as hormones, cancer, immobility, surgical procedures, long and confining travel, and a variety of other precipitating causes can result in a DVT. Recurrent DVT occurs in approximately 30% of patients during the first 10 years after the initial event.10 Although therapeutic anticoagulation is effective in preventing recurrent DVT, the duration of anticoagulation does not appear to affect the recurrence rate once the anticoagulation is stopped.9,10 For a subset of patients with idiopathic DVT, the disease may be considered chronic and require indefinite anticoagulation.11,12 Patients who develop DVT postoperatively and later have another operation have been shown by Borow and Goldson13,14 to have >60% chance of a recurrent DVT. If the first thrombosis was silent, then the surgeon may be unaware of this increased risk when the subsequent surgical procedure is done.
Another major complication of VTE is PTS, which includes any one or all of the following: leg pain, tenderness, leg fatigue, leg swelling, skin eczema, pigmentation, or open ulceration. The incidence of this long-term complication ranges from 7% to 82%, depending on the population studied and the length of follow-up. It has been estimated that 20% to 40% of these cases occur within 1 to 2 years after a DVT, and a severe form is seen in 5% to 10% of patients.15 Quality of life in patients with PTS is significantly worse than in those not affected by this syndrome.16 The 20-year cumulative incidence of venous ulcer was reported to be 3.7% in patients with past venous thrombosis according to a long-term population study.17 PTS represents a permanent and serious complication of DVT that results in enormous loss of productivity and substantial health care costs.18
The Clinical, Etiologic, Anatomic, and Pathophysiologic (CEAP) classification was developed more than a decade ago by an international panel of experts on venous disease, and this classification was used to categorize patients on the basis of their symptoms, physical findings, and diagnostic workup.19 This classification has been very helpful in grading venous disease, especially in those with PTS.
Prevention of Venous Thromboembolism
The importance of providing thrombosis prophylaxis for VTE cannot be overemphasized when one considers all of the potential complications described in the previous section. One should not determine the necessity from thrombosis prophylaxis solely based on preventing fatal PE, but in addition, to avert the many short- and long-term complications of VTE.
The vast body of data from prospective, randomized clinical trials shows the treatment of VTE uses various forms of prophylaxis compared with placebo, involving a treatment phase of 5 to 7 days followed by appropriate endpoint testing to calculate VTE incidence.20 Many of these studies were done when this period of hospitalization was common postoperatively. The current length of stay after many surgical procedures is much shorter, and sometimes procedures are even done as an outpatient basis, making postdischarge prophylaxis difficult. Some of these patients are as much in need of prophylaxis and have equal greater thrombotic risk than those patients in the older clinical trials. To complete a 5- to 7-day course of prophylaxis, these patients would have to receive daily injections, and in many situations, the costs would not be covered by insurance. Nevertheless, there are no data indicating that shortening the length of prophylaxis is justified simply because the patient is at home. In many cases, these patients are still relatively immobile and spend much time in recliners; this does not provide early ambulation, but rather, is a source of “early angulation” and may promote the development or extension of a leg thrombosis.
Evidence has emerged that reveals a significant incidence of VTE after discharge in patients having surgery. Agnelli et al21 undertook a prospective observational study of 2373 patients who underwent general (n = 1238), urologic (n = 685), and gynecologic (n = 450) surgery to evaluate the incidence of clinically overt VTE in patients who underwent cancer surgeries and to identify risk factors for VTE. Percentages of patients receiving prophylaxis dropped significantly postdischarge. Predischarge and postdischarge prophylaxis rates were 86.8% and 30.1% in general surgery patients, 82.7% and 29.5% in gynecologic patients, and 71.7% and 32.5% in urologic patients, respectively. In a multivariable analysis, five independent risk factors were identified: age >60 years, previous VTE, advanced cancer, anesthesia lasting more than 2 hours, and bed rest more than 3 days. These factors would be a good starting point for postdischarge prophylaxis after surgery and will be considered further in the section on Risk Assessment. Goldhaber et al22 reported on the results of a multi-institution observation study in patients who developed DVT. In this study, 40% of VTE and PE events occurred more than 21 days after surgery, leading researchers to conclude that there was a need to extend antithrombotic prophylaxis beyond hospital discharge and the conventional perioperative period. Merkow et al23 analyzed data from 211 acute coronary syndrome National Surgical Quality Improvement Program (NSQIP) hospitals and included 44,656 patients who underwent cancer surgical procedures; VTE occurred in 1.6% of all patients, most frequently after esophagogastric (4.2%) and hepatopancreaticobiliary (3.6%) surgery. Overall, 33.4% of VTEs occurred postdischarge (from 17.9% for esophagogastric to 100% for endocrine operations). Because one-third of VTE events in cancer surgery patients occurred postdischarge, the authors concluded that routine postdischarge VTE prophylaxis should be considered for high-risk patients.
Evidence-Based Guidelines versus Real Clinical Practice
Over the past 20 years, guidelines have appeared and have been refined to the point where they serve as a most valuable tool to assess the need and value of thrombosis prophylaxis. These guidelines are very specific because they are based on randomized clinical trials for the most part, and the recommendations are based on levels of evidence depending on the strength of the data.20 Unfortunately, these data are limited by the inclusion and exclusion criteria for the trials used to formulate the guidelines; therefore, many patients encountered in clinical practice would not be candidates for these trials. The authors of the American College of Chest Physicians (ACCP) document have commented on this, stating:
Prophylactic decisions for the individual patient are best made by combining knowledge of the literature with clinical judgment including detailed knowledge of that particular patient’s unique risks for thrombosis, the potential for adverse consequences due to the prophylaxis, and the availability of various prophylaxis options locally. The recommendations that are best for the group may not be the best for the individual.20
This crucial point has also been addressed by Diamond and Denton,24 who described alternative perspectives on the biased foundations of medical technology assessment.
Risk of Deep Venous Thrombosis
Risk Factors
The single most important factor in prevention of VTE is the identification of patients at risk. Considering all of the risk factors, a family history of thrombosis in a first-degree relative may be one of the strongest predictors of VTE and guide screening for relatives who are at high risk for VTE because of surgery or illness. Bezemer et al25 found in their studies that, “The risk increased with the number of factors identified; for those with a genetic and environmental risk factor and a positive family history, the risk was about 64-fold higher than for those with no known risk factor and a negative family history.” They further concluded that “family history is a risk indicator for a first venous thrombosis, regardless of the other risk factors identified. In clinical practice, family history may be more useful for risk assessment than thrombophilia testing.” Noboa et al26 studied this problem in almost 700 patients with a first-time thrombosis and matched controls, and concluded that “Family history of VTE could be added to a prior VTE history to define a concept of clinical thrombophilia which is not necessarily related to carrying a known inherited risk factor.”
Independent risk factors for VTE include advanced age; malignancy; cancer therapy, including chemotherapy, radiotherapy, or hormonal therapy; history of VTE; pregnancy and the postpartum period; estrogen-containing oral contraception or hormone replacement therapy; acute medical illness; heart or respiratory failure; inflammatory bowel disease; nephritic syndrome; myeloproliferative disorders; paroxysmal nocturnal hemoglobinuria; obesity; smoking; varicose veins; central vein catheterization; inherited or acquired thrombophilia; selective estrogen receptor modulators (raloxifene); surgery; immobilization; stroke with paralysis; and major or lower extremity trauma.20 Raloxifene, indicated for the prevention of osteoporosis in postmenopausal women, has been shown in a meta-analysis to be associated with a 62% increase in the odds of either a DVT or PE.27 Heit has pointed out that superficial venous thrombosis is a risk factor for subsequent VTE apart from the acute episode.9 Long-haul (>6 hours) air travel has also been associated with a slightly increased VTE incidence that is preventable with elastic stockings.28 Ray et al29 has reported that statin therapy may provide a 25% to 50% risk reduction for developing VTE. Heit in his review also mentions heparin-induced thrombocytopenia, disseminated intravascular coagulation and fibrinolysis, thromboangitis obliterans, thrombotic thrombocytopenic purpura, Bechet’s syndrome, systemic lupus erythematosus, homocysteinuria, and perhaps, hyperhomocysteinemia. Recently, a study appeared documenting the incidence of VTE in those with diabetes.30 In 2488 consecutive patients, 476 (19.1%) had diabetes and were more likely than patients without diabetes to experience recurrent DVT (14.9% vs 10.7%; P = 0.01) as well as long-term major bleeding complications (16.4% vs 11.7%; P = 0.01). Patients with diabetes who developed VTE were more likely to have a complicated clinical course. The authors also concluded that diabetes was an independent predictor of VTE and the rate of thromboprophylaxis in these patients was low.
Another important risk factor affecting pregnant women is the antiphospholipid syndrome, including a history of unexplained stillborn infants, recurrent spontaneous abortions, premature birth with toxemia, or a growth-restricted infant. The diagnosis is established by detecting antibodies against phospholipid-binding proteins or a circulating anticoagulant on laboratory testing.31 The hematologic abnormality may exist long after pregnancy and represents a significant risk factor for VTE. Patients should be carefully questioned about these obstetric events in order to not overlook an occult thrombophilia defect that, if present, may greatly increase the VTE risk in hospitalized medical or surgical patients. Further complicating this analysis is the fact that all risk factors are not of equal weight as far as the incidence of VTE is concerned. It is well known that with advancing age, the VTE incidence rises to about 1% of the population over the age of 75 years.32
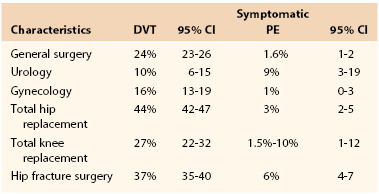
Surgery is a key exposing risk factor for developing VTE. Table 51-1 shows the expected incidences of VTE in patients without prophylaxis. Borow and Goldson13,14 have shown that the incidence of VTE dramatically increases with age and length of surgery. Sugerman et al33 have reported that morbid obesity (body mass index [BMI] >55 kg/m2) associated with the venous stasis syndrome, characterized by leg swelling and chronic skin changes, is associated with a very high VTE incidence, including an increased fatal PE rate compared with those with a BMI <55 kg/m2. Cancer patients undergoing surgery have a two- to fivefold increased risk for postoperative VTE.34,35 Kroger et al36 has shown the effect of multiple risk factors on the incidence of VTE in cancer patients. These factors included inpatient treatment, previous DVT, a family history of DVT, chemotherapy, fever, and elevated C-reactive protein (CRP). The VTE incidence in the absence of all of these factors was 2.3%, whereas it was 72% when all seven factors were present. Anderson and Spencer37 have shown in a community health study in Massachusetts that the risk of DVT increases with the number of risk factors, and patients with five or more risk factors who are in hospital and have not received prophylaxis had a 100% chance of developing VTE. This landmark study, along with the work of Heit, demonstrates the importance of assessing individual patient risk over and above the group recommendations for various types of surgery or medical illnesses.9 Failure to include important risk factors due to an inadequate history and physical examination relative to VTE may underestimate the patient’s risk.38 Without a detailed knowledge of the individual patient’s thrombotic risk, a proper informed consent relative to this risk may not be possible. In addition, the intensity and type of thrombosis prophylaxis used by the surgeon may require modification due to the level of risk. Extended out-of-hospital prophylaxis may be indicated and powerful anticoagulants that may increase the chance of bleeding. These are all issues that need to be discussed with each individual patient.39
Risk Assessment Models
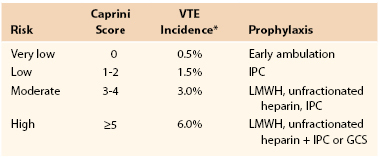
Appropriate thrombosis prophylaxis requires tailoring the prevention to the level of risk. Low-risk patients do not need prophylaxis, whereas those at the highest risk may need the most effective combinations demonstrated by clinical trials.39 It is critical to make individual patient decisions based on a complete history and physical examination designed to uncover all possible risk factors. We have created and validated a simple risk factor assessment tool based on assigning a point score to each common risk factor. This score has been linked to the incidence of clinically significant VTE at 30 and 60 days in a variety of patient groups, including general and vascular surgery, plastic surgery, otolaryngology, and a study of mixed medical and surgical patients. More than 17,000 patients have been studied in these trials.40–45 It has been shown that the number one preventable cause of death in hospitalized patients is VTE.46 It seems that screening for this disease should be the first priority for hospitalized patients, and assessments that are routinely done on admission for fall and bedsore risk should take second place to VTE risk assessment. Table 51-2 shows the incidence of clinically relevant VTE events in patients without prophylaxis based on the Caprini score (Fig. 51-1). A score of at least 5 is associated with a 6% clinically relevant VTE incidence. These data have been compiled by the editors of the 2012 ACCP guidelines, who recommend using this score as one option for assessing risk and deciding on the use of thrombosis prophylaxis.47 An additional facet of the score is that those with a score >8 were found to have an incidence of VTE of 6.5% in general and vascular surgery patients at 30 days postdischarge. Patients who underwent elective plastic surgical procedures had an 11.3% rate 60 days after surgery, and for head and neck procedures, the 30-day incidence was 18.3% when the score was >8. This ultra-high subset of patients may benefit from prolonged prophylaxis for days or weeks following discharge, but further studies will be necessary to learn how to provide prophylaxis for this group.
Methods of Prophylaxis
As demonstrated by Rudolph Virchow over 150 years ago, pathogenesis of DVT is related mainly to hypercoagulability, venous stasis, and endothelial damage. Hypercoagulability is very common during the postoperative period as a body’s response to the surgical aggression, and in patients with trauma, sepsis, cancer, and hereditary thrombophilia. In contrast, venous stasis plays a major role because it prevents the local clearance of activated coagulation factors and reduces their binding with physiologic inhibitors. In addition, when associated with venous dilatation, stasis may alter the normal linear blood flow along the platelets, red blood cells, and white blood cells to contact the endothelium. Some studies have shown significant dilatation of the leg veins during surgery, which may result in endothelial cracking and exposure of the procoagulant subendothelial collagen and other molecules, such as vitronectin and fibronectin, resulting in thrombosis.48 Vascular damage may also occur in patients undergoing surgery close to the proximal deep veins, as is the case during hip and knee replacement, varicose vein surgery, trauma, and pelvic surgery for tumors.
For the previous reasons, most available methods to prevent VTE are either mechanical, aiming to improve venous flow and reduce stasis, or pharmacologic, based on agents that act as anticoagulants to counteract hypercoagulability. These methods may be used separately or combined, depending on their availability and the potential thrombotic and hemorrhagic risk of the patient.
General Measures
Apart from the usual measures taken during the postoperative period, such as adequate hydration and analgesia, it is very important that patients start to ambulate as soon and as much as possible. For those patients who are immobile in bed, repeated active flexo-extension exercises of the ankle joints should be encouraged to enhance venous flow. The assistance of nurses and auxiliary personnel is extremely valuable to achieve a good implementation of these actions.
Leg elevation has a dual physiologic effect: it reduces swelling and improves venous return and reduces venous pressure by its gravitational effect. From this point of view, it is probably better being in bed with the legs elevated than being seated in an armchair We should not confound early ambulation with “early angulation,” because the fact that patients are seated does not necessarily mean that they ambulate enough.
Mechanical Methods of Prophylaxis
Although mechanical methods of prophylaxis have not been studied as extensively as the pharmacologic agents that are discussed in the following, they often represent the only alternative for patients with contraindications to receive anticoagulants. Most physical methods may be combined with the pharmacologic agents in patients at very high risk to develop VTE, because they act on different pathogenic factors, as explained in the previous section.
The preferred modalities are based either on passive compression of the legs with graduated elastic stockings or active compression, such as intermittent pneumatic compression of the legs and foot impulse devices, also known as foot pumps. Apart from reducing stasis, some of these methods also stimulate endothelial fibrinolytic activity.49
Elastic Compression Stockings
Graduated compression stockings (GCSs) reduce the cross-sectional area of the veins and increase the velocity of venous blood flow. This acceleration of blood flow depends on the pressure gradient applied from the ankle to the knee or thigh level, and the GCSs provide some gradient effect.50 In addition, GCSs prevent intraoperative distention of the calf veins in patients receiving general anesthesia.51,52 It is very important to differentiate GCSs used for primary prophylaxis of DVT from therapeutic graduated stockings, which apply a pressure of 30 to 40 mm Hg at the calf and are used for the secondary prevention of PTS after a DVT is diagnosed or as therapy once this syndrome arises.
Recently, new data have appeared that cast doubts on the effectiveness of GCSs in stroke patients. The first study compared the use of thigh-length GCSs versus no stockings in immobile stroke patients. The authors found no change in the DVT incidence between the groups. DVT occurred in 10% of patients allocated to thigh-length GCSs and in 10.5% in the no-stocking group.53 These differences were not statistically significant. Skin breaks, ulcers, blisters, and skin necrosis were significantly more common in the GCS group (5.1%) compared with those without hose (1.3%). A second study was done in immobilized stroke patients comparing thigh-length versus calf-length GCSs, and the results showed that thigh-length GCSs were more effective than below-knee stockings.54 Proximal DVT occurred in 6.3% who received thigh-length stockings and 8.8% in those who received below-knee stockings (P = 0.008, an odds reduction [OR] of 31%; 94% confidence interval [CI], 9%-47%). It is not known how these results translate to the surgical population.
A systematic review from the Cochrane Collaboration analyzed seven randomized controlled trials in surgical patients. The incidence of postoperative DVT was significantly reduced from 29% in the control group to 15% in the GCS group (P < 0.001) (OR, 0.33; 95% CI, 0.26-0.49).55 Similarly, a meta-analysis of 11 studies investigated the efficacy of GCSs in moderate-risk patients who underwent mainly abdominal surgery. The results indicated a significant 68% reduction in the rate of postoperative DVT (P < 0.0001) (OR, 28; 95% CI, 0.23-0.48).56 Other reviews have documented reductions in the incidence of DVT between 57% and 66%.57
Current evidence suggests that GCSs used alone are effective in moderate-risk general surgical patients, but there is a lack of data in high-risk patients, such as cancer or major orthopedic surgical patients. Also, there is no evidence indicating that GCSs reduce the incidence of PE in medical or surgical patients.
Limitations of GCSs include lack of international standardization of the stockings’ pressure profiles, difficulty in their application in patients with unusual leg shapes or sizes, and poor compliance by some patients. In addition, they should not be used in patients with peripheral arterial disease, lack of foot pulses, or an ankle-brachial index less than 0.8. Stockings are also contraindicated in patients with massive leg edema, especially if associated with congestive cardiac failure, and in patients with dermatitis.
One benefit of GCSs is the possibility to combine them with pharmacologic methods in patients at very high risk to develop VTE. There is always a tradeoff between improving prophylaxis versus causing skin problems.
Intermittent Pneumatic Compression
Intermittent pneumatic compression (IPC) of the lower limbs represents the most extensively studied of the mechanical modalities of thrombosis prophylaxis and is considered the most effective. Basically, most IPC devices consist of pneumatic boots or garments that are wrapped around the legs. The boot or sleeve is connected to an electrical compressor that intermittently insufflates air to a preselected pressure. Hemodynamic studies have shown that uniform IPC might trap blood in the distal veins of the leg, whereas intermittent sequential compression would not, thus enhancing venous return.58
IPC avoids venous stasis by intermittent pumping of the leg veins. The maximum pressures utilized by most investigators range from 35 to 55 mm Hg, and inflation times vary from 10 to 35 seconds, with a deflation period of approximately 1 minute, to allow the veins to refill with blood between the compression cycles. A device has been designed that has the ability to detect the change in the venous volume of the legs and to respond by initiating the subsequent compression cycle when the veins are substantially full.59
Apart from improving venous hemodynamics, IPC stimulates fibrinolytic activity, increasing the blood levels of prostacyclin and tissue-type plasminogen activator (tPA).60 Moreover, IPC also increases the levels of tissue factor pathway inhibitors.61 The results of the trials conducted to assess IPC have been variable, depending on the type of surgery performed, patient risk factors, and endpoint used to detect DVT. Overall, most studies show that this method is effective in preventing postoperative DVT detected by objective diagnostic methods in patients undergoing general surgery,13 neurosurgery,62 gynecologic,63,64 urologic,65 and orthopedic surgery.66 In a recent meta-analysis that examined 19 trials in 2255 patients assessing IPC as monotherapy, this modality of prophylaxis produced a highly significant 66% reduction in DVT from 10% in the IPC group to 23.4% in the control group (P < 0.00001).67 This reduction was similar with uniform and sequential compression devices. Likewise, another meta-analysis of 15 studies including 2270 patients who underwent different types of surgery showed that, in comparison to no prophylaxis, IPC devices reduced the risk of DVT by 60% (relative risk, 0.40; 95% CI, 0.29-0.56; P < 0.001).68
Of the available mechanical methods, IPC is the most effective in orthopedic surgery; however, the incidence of proximal DVT is higher than with some pharmacologic methods, such as the low-molecular-weight heparins (LMWHs). In this regard, two meta-analyses have shown that IPC and warfarin, a vitamin K antagonist, (VKA), represent good options for DVT prophylaxis in hip replacement surgery and is similar to LMWH and warfarin in knee replacement.69,70 Regarding the timing of use in surgical patients, IPC should start immediately before surgery, and continue until the patient returns to usual level of mobility.20 During admission to the ward, IPC should be used as much as possible while the patient is in bed or sitting in a chair.
Whether above-knee or thigh-length devices are better than below-knee or calf-length sleeves remains controversial because this issue has not been addressed in large-scale trials. Therefore, according to a systematic review, the choice between both modalities may be done on practical grounds, depending on their availability and cost.67 IPC is particularly useful in patients at high risk of bleeding complications, such as neurosurgery, pelvic surgery, and severe trauma cases, or when pharmacologic agents are contraindicated (Box 51-1). In this regard, a recent epidemiologic study involving over 65,000 patients worldwide has revealed that 9% of all surgical patients at risk have some contraindication to receive prophylaxis with anticoagulants, and therefore, would be good candidates for mechanical prophylaxis.71 A recent study in 395 patients compared 40 mg of enoxaparin with 81 mg of aspirin daily for 10 days following total hip replacement. Both groups used a portable calf IPC device for a mean of 20 hours daily. Major bleeding events were 0% using compression and aspirin compared with 6% using LMWH. The rate of distal DVT in both groups was 3%; proximal DVT was seen in 2% of those using aspirin and compression and in 1% of the LMWH group.72 The ninth edition of the ACCP guidelines recommends using IPC in surgical patients at high risk for VTE and increased risk for bleeding.47
Some adverse effects of IPC have occasionally been reported, such as skin irritation, peroneal nerve palsy, and skin pressure necrosis. However, most of these problems were attributed to improper placement of the sleeves. IPC should be avoided in patients with skin infections such as cellulitis and erysipelas, and in patients with massive edema of the legs secondary to congestive heart failure because of the risk of excessively increasing the preload on the heart. IPC is contraindicated in patients with established DVT because the thrombus could be partially or completely detached from the venous wall and embolize to the lungs. Therefore, the presence of a DVT should be ruled out before applying these devices to patients at high risk with previous immobilization. The presence of peripheral arterial disease may be another contraindication for the use of IPC because of the risk of compromising tissue viability, resulting in skin necrosis.
Another limitation of IPC is the low compliance by nurses, in which the correct use of IPC ranged from 29% to 78%.73,74 In a recent study, half of the patients not wearing the IPC sleeves indicated that the reason was their removal after recent ambulation or bathing.74 These studies clearly highlight the need for ongoing staff and patient education on the correct use of IPC to improve prophylaxis. The seventh edition of the ACCP guidelines recommended paying special attention to ensure the proper use and optimal compliance with the mechanical devices for VTE prevention.20
Foot Compression Devices
These devices, also known as foot pumps, consist of inelastic slippers or boots with an air bladder in the area of the sole of the foot. This air chamber is rapidly inflated to a pressure up to 200 mm Hg over 3 seconds every 20 seconds. This plantar compression increases venous outflow and reduces stasis in the legs, and therefore, has been investigated for the prevention of DVT. Several studies were conducted showing a reduction in venographically detected DVT in orthopedic patients with the use of the foot pump compared with no prophylaxis. A systematic review of two trials showed that foot compression reduced the risk of DVT by 77%; however, there were no significant differences in the rates of PE or proximal DVT.67 Another study found similar results of foot compression and LMWH after THR.75 Several trials reported better results with LMWHs than with foot compression in patients who underwent knee replacement.76,77 Accordingly, there are limited data demonstrating a beneficial effect of the foot compression device in surgical patients. However, patients at high risk of VTE in whom the use of anticoagulants is contraindicated and IPC cannot be used, such as trauma cases with external fixation in the legs, may be suitable candidates to receive foot compression as an alternative.
Pharmacologic Methods of Prophylaxis
As mentioned previously, hypercoagulability represents a key risk factor for the development of VTE. Therefore, several pharmacologic agents with different anticoagulant actions have been evaluated for VTE prevention during the last 40 years. These methods include subcutaneous unfractionated heparin, LMWH, dextrans, and oral VKAs, among others. More recently, new promising molecules that inhibit some specific components of the clotting cascade have been developed, and some have received approval by health regulatory agencies. See Chapters 34 and 35 for additional discussion of these drugs.
Unfractionated Heparin (UFH)
Low-dose (5000 U given 2 hours before surgery followed by 10,000-15,000 U/24 h) subcutaneous UFH was the first pharmacologic agent to be widely investigated for the primary prevention of VTE in patients undergoing surgery. In a landmark multicenter international study, Kakkar et al78 demonstrated that low-dose unfractionated heparin significantly reduced the risk for both DVT and PE in general surgical patients, including fatal PE. Unfractionated heparin saved 7 lives for every 1000 patients receiving it.
Several meta-analyses of the literature confirmed that unfractionated heparin reduces the rate of postoperative DVT by more than 50% in general surgical patients with minor bleeding complications.79 A systematic review of 75 studies including 16,000 patients compared unfractionated heparin versus no prophylaxis in surgery. The results of this review showed that unfractionated heparin significantly reduced the risk of DVT by 56% compared with no prophylaxis (risk ratio [RR], 0.44; 95% CI, 0.37-0.52) and the risk of PE by 30% (RR, 0.70; 95% CI, 0.53-0.93) without significantly increasing the risk of bleeding complications.80 Although no direct comparisons have been made, in general, unfractionated heparin (5000 IU) should be given twice daily in moderate-risk patients, and three times daily in high-risk patients. Some reviews have documented an increase of wound hematomas from 3.8% to 6.2% that could be minimized by avoiding subcutaneous injection of heparin near surgical wounds.
Unfractionated heparin is very effective for the prevention of DVT and PE in general surgery, gynecology, and urology. However, the efficacy of this method is lower in patients undergoing major orthopedic surgery, such as elective hip and knee replacement. In contrast, unfractionated heparin has been associated with heparin-induced thrombocytopenia in up to 5% of patients receiving heparin. This disorder is mediated by the development of antiplatelet antibodies and characterized by a dramatic drop in the platelet count, usually below 100,000 mm3. In 20% of patients with heparin-related thrombocytopenia, there is thrombosis that may lead to ischemic complications.81 The short half-life of unfractionated heparin (0.5-2 hours) is another limitation because it makes more frequent dosing necessary. Yet, this short half-life may be advantageous in cases of bleeding complications or in patients with renal failure. Another advantage of unfractionated heparin is the possibility of rapid reversal of its anticoagulant action with protamine sulfate.
Low-Molecular-Weight Heparins (LMWHs)
Although unfractionated heparin represented the standard for pharmacologic VTE prevention for over a decade, in the 1980s, a new group of heparin fractions, generically known as LMWHs, with an average molecular weight between 4000 and 8000 Da, were developed and accepted for clinical use. These fractions have greater activity against factor Xa than against factor II, whereas these actions are similar with unfractionated heparin. This effect could explain the experimental finding that LMWHs were able to prevent venous thrombosis without increasing bleeding risks. In addition, LMWHs have a better bioavailability and longer half-life than unfractionated heparin when administered subcutaneously. Because of their lower binding to the endothelial cells, macrophages, and circulating proteins, LMWHs have a more predictable anticoagulant response than unfractionated heparin. For the previous reasons, LMWHs have practical advantages for their clinical use, including the possibility of a single daily injection without the need for laboratory monitoring, which makes them ideal for outpatient prophylaxis.20
The efficacy of LMWHs for the prevention of postoperative VTE has been demonstrated in several randomized controlled trials and meta-analyses comparing LMWHs with placebo and unfractionated heparin in general and with unfractionated heparin or warfarin in major orthopedic surgery.82–87
In the meta-analysis conducted by Mismetti et al,85 LMWHs achieved a significant reduction in the incidence of asymptomatic and symptomatic VTE in general surgery, compared with placebo or no treatment. Similarly, the comparison between LMWHs and unfractionated heparin showed a trend in favor of LMWHs, with a significant reduction in clinical VTE. LMWHs at doses less than 3,400 anti-Xa U per day seemed to be as effective and safer than unfractionated heparin, whereas higher doses resulted in higher efficacy but increased bleeding risk. However, as shown by Bergqvist et al,88 in very high-risk patients, such as in those with cancer, higher doses of LMWHs may offer increased efficacy without increasing bleeding risk. This may be the result of the effect of hypercoagulability associated with cancer, which in some way might protect these patients from bleeding.
In major orthopedic surgery, particularly in total joint replacement, LMWHs have been extensively investigated and compared with unfractionated heparin and warfarin. Over 20 years ago, some trials showed that LMWHs were superior to unfractionated heparin for the prevention of postoperative VTE following THR without a significant increase in bleeding complications.89 These findings have been confirmed by subsequent meta-analyses.83 In contrast, studies comparing LMWHs with warfarin in these patients showed that the heparin fractions were more effective, but were also associated with a higher bleeding risk.90 Because LMWHs are easier to administer than oral VKAs because no monitoring is needed, they have become the most used pharmacologic method for patients undergoing THR, particularly in Europe, as shown in several surveys and epidemiologic studies.71
In patients undergoing total knee replacement, several trials show that LMWHs are superior to oral anticoagulants, although the rate of DVT remains unacceptably high (between 20% and 30%).91 A meta-analysis from the United States indicates that LMWHs are a better alternative than warfarin.92 Patients with hip fractures are at very high risk of VTE, and accordingly, should receive appropriate prophylaxis. Unfractionated heparin and LMWHs are better than placebo in reducing postoperative DVT in this population.93
Several trials have been conducted in medical patients admitted to hospital. Heparin and LMWHs reduced the incidence of DVT by 70% compared with no prophylaxis without a significant increase of bleeding.20 Another trial, Medical Patients with Enoxaparin (MEDENOX), compared enoxaparin, either 20 or 40 mg subcutaneously once daily, with a placebo in more than 1000 medical patients admitted to hospital because of acute conditions such as congestive heart failure, acute respiratory failure, and acute infection. The rates of DVT, detected by venography in most patients, were 14.9%, 15%, and 5.5% in patients receiving placebo, 20 mg of enoxaparin, and 40 mg of enoxaparin, respectively.94 Similar results have been reported in another trial comparing LMWH (dalteparin 5000 U/day) with placebo.82 The rates of symptomatic VTE and asymptomatic DVT detected by ultrasonography by day 21 were 2.8% in the treated and 5% in the placebo group, respectively (P = 0.002).
In summary, LMWHs have become the standard of care for the prevention of VTE in most countries for surgical and medical patients. Although these heparin fractions are described collectively as LMWHs, each of these products has different biologic and clinical properties. Therefore, the experts and the regulatory agencies, such as the U.S. Food and Drug Administration (FDA), consider LMWHs to be distinct drugs requiring indications and dosage specifications for each product. Accordingly, because of these differences, different LMWHs should not be therapeutically interchanged.95
Oral Vitamin K Antagonists
Coumarin derivatives such as warfarin and acenocoumarol act as vitamin K antagonists (VKAs). For the past 50 years, several VKAs have been used for the prevention and management of VTE. The most commonly used VKA in North America is warfarin. This product can be administered orally, either at fixed low doses that do not need laboratory monitoring, or at adjusted doses, aiming to achieve a therapeutic level of anticoagulation, using the international normalized ratio (INR). A prolongation of the prothrombin time corresponding to an INR between 2.0 and 3.0 is considered adequate for VTE prophylaxis in high-risk patients.
Because most VKAs need 3 to 4 days to achieve their maximum anticoagulant effect, these products are usually started on the evening of the operation or on the first postoperative day, aiming to obtain the previous therapeutic INR level around the third or fourth postoperative day and adjusting the dose by serial laboratory monitoring during the first postoperative days.
The main advantage of VKAs is the possibility for oral administration, which make them suitable for use after hospital discharge. The main disadvantages are the risk of bleeding, the need for frequent monitoring, and many interactions with other drugs and diet. The anticoagulant effect of VKAs can be reversed with vitamin K, prothrombin concentrates, and fresh frozen plasma.
The efficacy of VKAs, particularly of warfarin, for the prevention of VTE in orthopedic surgery has been shown in several clinical trials and systematic reviews of the literature. Overall, VKAs reduce the risk of postoperative DVT by 50% (RR, 0.49; 95%, CI 0.34-0.73) compared with no prophylaxis, but these drugs also significantly increase the risk of bleeding complications by 58% (RR, 1.58; 95% CI, 1.01-2.47). Conversely, LMWHs are more effective compared with VKAs, because the risk of DVT is 43% higher than with LMWHs (RR, 1.43; 95% CI, 1.31-1.56).67
In summary, VKAs are effective in reducing VTE but increase the risks of major bleeding. Adjusted doses with INR monitoring are more effective than fixed low doses for the prevention of DVT. Although warfarin remains a popular modality of prophylaxis among orthopedic surgeons in the United States, they have been replaced by LMWHs in Europe.96
Aspirin
Aspirin (acetyl salicylic acid) inhibits platelet function through its irreversible inhibition of the enzyme cyclooxygenase (COX-1), thereby blocking platelet aggregation. Regarding prevention of VTE, the role of aspirin remains somewhat controversial. One systematic review compared the efficacy of aspirin and other antiplatelet products with no prophylaxis, finding a modest reduction in the incidence of DVT, around 34% in nine studies (RR, 0.69; 95% CI, 0.48-0.97).97 This review did not find significant differences in the rates of bleeding complications between aspirin and no prophylaxis (RR, 1.30; 95% CI, 0.67-2.52).
A clinical trial has documented a significant reduction in the incidence of symptomatic VTE with use of aspirin (160 mg) daily compared with placebo in patients operated for THR and hip fracture.98 For this group, the reductions were 43% and 29% for PE and DVT, respectively. The results of this study should be analyzed with caution because other modalities of prophylaxis (stockings, heparin, LMWH) were used in 40% of the patients. Furthermore, aspirin was effective only in the hip fracture patients but not in those who underwent THR. The rates of wound-related and gastrointestinal bleeding were significantly higher in patients receiving aspirin. Although previous editions of the ACCP guidelines gave a strong recommendation against the use of aspirin for VTE prevention in major orthopedic surgery, a reassessment of the preceding trial by the 12th edition of the guidelines resulted in a new recommendation in favor of aspirin in patients undergoing major orthopedic surgery. However, these guidelines suggest the use of LMWH in preference to aspirin.99
New Antithrombotic Anticoagulants
Currently available antithrombotic agents have several limitations: insufficient efficacy in some high-risk patient populations, such as those with hip and knee arthroplasty, parenteral administration with subcutaneous injections, risk of heparin-induced thrombocytopenia, and for VKA, the need for repeated monitoring and dose adjustments and the narrow therapeutic window. Therefore, major efforts are being made to develop new antithrombotics with higher efficacy, more specific action by inhibiting single enzymes, and that are more convenient to use. Eventually, some of these new agents will gradually replace LMWHs and VKAs. These antithrombotics, some of them already approved, may be classified according to the level at which they act in the coagulation cascade. For more information, see Chapter 34.
Factor Xa Inhibitors
Indirect: Fondaparinux.
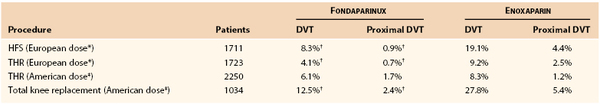
Fondaparinux is a synthetic pentasaccharide that selectively inhibits factor Xa by producing a conformational change in the natural anticoagulant antithrombin molecule. Given by subcutaneous injection, fondaparinux has a very high bioavailability and a half-life of 17 hours. Contrary to heparin, fondaparinux does not cause thrombocytopenia, and no osteoporosis has been observed in patients who were treated with this product. The clinical efficacy of fondaparinux, started 6 to 8 hours after the end of surgery, has been investigated in several randomized, double-blind controlled trials in patients undergoing THR, total knee replacement, and hip fracture surgery. Four trials included more than 7300 patients in whom fondaparinux was compared with enoxaparin in patients who underwent these operations (Table 51-3). Overall, the combined data suggest that fondaparinux prophylaxis reduces postoperative DVT detected by routine bilateral venography and symptomatic PE by 50%, with a similar risk of bleeding.100 Another trial compared 7 days of fondaparinux with 30 days in patients after a hip fracture with bilateral venography performed between 19 and 24 days after discharge. The differences were striking, because the DVT rates were 35% and 1.4% in patients who received fondaparinux at 7 and 35 days, respectively. Furthermore, the incidences of symptomatic VTE were 2.7% and 0.45%, respectively.101 The U.S. FDA has approved fondaparinux for the aforementioned indications.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree