Acute Aortic Occlusion (Open Surgery)
PANTELIS HADJIZACHERIA, ROBERT S. CRAWFORD, and RAJABRATA SARKAR
Presentation
A 62-year-old man with a history of hypertension, hyperlipidemia, peripheral vascular disease, and a 90-pack-year history of smoking presents to the emergency department with bilateral lower extremity paresthesia, pain, and decreased motor function. He denies back pain or abdominal pain, and has a prior history significant for severe bilateral claudication worsening over the last 2 years and occurring at a distance of less than half a block. His vital signs are normal. Pertinent findings on physical examination are the absence of palpable femoral, popliteal, posterior tibial, and dorsalis pedis arterial pulses bilaterally. He has decreased sensation and strength in both lower extremities.
Differential Diagnosis
The conditions that mimic acute aortic occlusion include arterial dissection, traumatic spinal column/cord injury, lumbar disk herniation, transverse myelitis, acute stroke, and diffuse thromboembolism. In this patient with acute bilateral lower extremity pain and numbness, cardiovascular risk factors, and absent bilateral femoral pulses, the primary diagnosis is acute aortic occlusion until proven otherwise.
Workup
In the evaluation of the patient with acute leg symptoms, the duration and severity of leg symptoms coupled with lower extremity pulselessness points to a vascular cause requiring immediate treatment. The symptoms of aortic occlusion are reflected in the physical findings of lower extremity arterial ischemia and include the “5 Ps”: pain, pulselessness, pallor, paresthesia, paralysis, and poikilothermia. Nonvascular causes of similar symptoms listed above in the differential diagnosis will not result in pulselessness, a key differentiator in the evaluation of the patient.
All patients with suspected acute aortic occlusion should be investigated emergently with imaging to define the cause of occlusion and plan revascularization. The following additional studies should be obtained in patients with acute aortic occlusion: electrocardiogram, standard chemistry, complete blood count, prothrombin time, partial thromboplastin time, creatinine phosphokinase, and myoglobin level.
Arteriography is traditionally used for localizing an obstruction and visualizing the distribution of disease before aortoiliac revascularization. However, due to the increased availability, resolution, and speed of computerized tomography (CT), CT angiography (CTA) is the test of choice in the acute setting. Our patient underwent further evaluation with a CT scan that revealed complete occlusion of the infrarenal abdominal aorta and reconstitution of flow at the level of the right and left common femoral arteries (Figs. 1 and 2). However, a major drawback of CTA is the hampered vessel assessment by arterial wall calcifications. Bedside arterial duplex can efficiently detect the femoral or iliac occlusion, which often shows no flow, or monophasic waveforms distally with decreased distal velocities. In the acute setting, the ability of duplex scan to consistently visualize the aorta is poor given bowel gas and other confounding factors. CT scanning is more widely and rapidly available in hospitals and is not operator or patient dependent, making it the ideal test for suspected aortic occlusion.
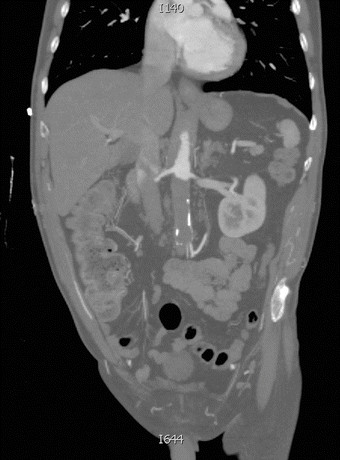
FIGURE 1 Coronal cut of a CT scan demonstrating complete occlusion of the infrarenal abdominal aorta.
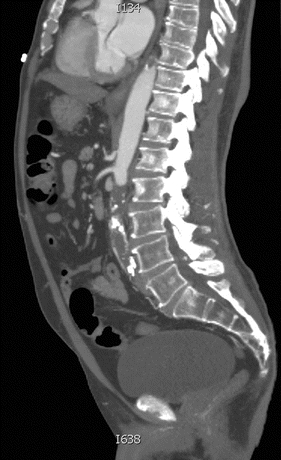
FIGURE 2 Sagittal cut of a CT scan demonstrating complete occlusion of the infrarenal abdominal aorta.
Discussion
Acute aortic occlusion is a rare occurrence; however, it is a true vascular emergency that requires rapid diagnosis and aggressive surgical intervention to limit poor outcomes. The two main causes for acute aortic occlusion to occur are arterial embolism and thrombosis. Determining associated comorbidities, such as arrhythmias or prior peripheral vascular disease, can help distinguish the two pathologies and guide management. In embolic aortic occlusion, the source of the embolus is almost invariably cardiac in origin and it can be catastrophic because patients tend to lack preexisting collaterals. Patients often recall the exact moment of onset of severe ischemic symptoms. In contrast, the less common acute aortic thrombosis is the result of progressive atherosclerotic narrowing in patients with aortoiliac occlusive disease, with a superimposed acute occlusion when flow decreases below the threshold required to maintain patency. Often, there is an antecedent recent history of generalized illness or dehydration that promotes hemoconcentration and hypercoagulability. Because of the prior presence of collateral vessels in long-standing aortoiliac occlusive disease, the clinical manifestations are seldom as dramatic as those of embolization as patients note that symptoms develop gradually over several days.
Diagnosis and Treatment
The most important aspect of diagnosis of acute aortic occlusion is to identify vascular occlusion as the cause of lower extremity neurologic symptoms. The authors have experienced several cases in which the symptoms were attributed to other causes and the aortic occlusion was identified 24 to 72 hours later. These cases uniformly end in bilateral amputation and/or death.
Initial goal of treatment for acute aortic occlusion is to prevent thrombus propagation and worsening ischemia; therefore, intravenous loading dose (100 U/kg) heparin should be administered immediately unless contraindicated. The critical decision in the treatment of acute aortic occlusion was traditionally to determine whether the etiology was an embolus or thrombosis. The rationale for this was that embolus was treated with emergent transfemoral embolectomy and thrombosis required contrast arteriography to define the arterial anatomy. This early distinction has vanished with the widespread use of CTA as described above, which readily distinguishes the two disorders.
The options for open revascularization in patients with aortoiliac occlusion are urgent direct or extra-anatomic arterial revascularization. Selection of the most appropriate method depends largely on the patient’s surgical risk, comorbid medical conditions, and degree of extremis. The potential options include direct aortofemoral bypass or extra-anatomic axillobifemoral bypass and femorofemoral bypass. Patients presenting with hypovolemia, acidosis, or oliguria should not be subjected to the greater operative stress of aortofemoral bypass. In selected cases, endovascular revascularization with recanalization, angioplasty, and stenting can be used to rapidly reestablish arterial inflow.
TABLE 1. Acute Aortic Occlusion (Open Surgery)

Surgical Approach to Aortofemoral Bypass
In a good-risk patient without evidence of hemodynamic compromise or advanced ischemia, emergent aortofemoral bypass is the procedure of choice for acute aortic occlusion secondary to thrombosis. The patient is widely prepped and draped from nipples to knees. The femoral vessels are preferentially exposed before making the abdominal incision to minimize the length of time that the abdomen is open. The infrarenal abdominal aorta may be exposed using a midline incision; however, a retroperitoneal approach may be employed in obese patients or those with a hostile abdomen.
The groin incisions are extended slightly above the inguinal ligament, and the common femoral, superficial femoral, and profunda femoris arteries are dissected in preparation for clamping, and retrograde retroperitoneal tunnels are begun. A midline abdominal incision is then created extending from the xiphoid to the pubis. After careful exploration of the intra-abdominal organs, the transverse colon and omentum are elevated and retracted cephalad, and the entire small bowel is displaced to the right. A self-retaining retractor is placed. Thereafter, the posterior parietal peritoneum overlying the infrarenal aorta is incised and extended cephalad where the ligament of Treitz is divided. This allows mobilization of the fourth portion of the duodenum off the aorta and facilitates visualization of the left renal vein. The inferior mesenteric vein can be divided if needed, and the aortic dissection is extended distally just beyond the origin of the inferior mesenteric artery. Next, retroperitoneal tunnels are made anterior to the common and external iliac vessels and posterior to the ureter for passage of each graft limb from the aorta to the groins. A Penrose drain or Silastic tubing is drawn through the tunnel.
A prosthetic graft of choice is used for construction of the bypass. For the proximal aortic anastomosis, an end-to-end anastomosis is typically preferred for aortic occlusion and in the setting of concomitant aneurysm disease, juxtarenal aortic occlusion, or the presence of a coral reef aorta. The indications for an end-to-side proximal anastomosis are occluded or severely diseased external iliac arteries, a sizable accessory renal artery arising from the infrarenal aorta, and the presence of a large inferior mesenteric artery. These conditions can be readily discerned on preoperative CTA.
After intravenous administration of heparin (100 U/kg) for an activated clotting time (ACT) greater than 250, appropriate vascular clamps are applied to the aorta just caudal to the renal arteries and immediately caudal or cephalad to the inferior mesenteric artery. For an end-to-end anastomosis, the aorta is transected and a 3- to 4-cm-long segment between the clamps is resected to allow the prosthetic graft to lie without angulation or protrusion in the retroperitoneum. Any patent lumbar artery branches arising from this segment are clamped and ligated. The transected distal aortic end is oversewn in two layers with monofilament suture. Thrombus or debris is removed from the proximal divided end of the aorta, and a proximal anastomosis to the graft is constructed with monofilament suture. Teflon pledgets may be used to bolster the suture. After completion of the aortic anastomosis, the graft is clamped with an atraumatic vascular clamp (Fogarty soft-jawed clamp) and the proximal anastomosis tested by slow release of the proximal aortic clamp. Next, the previously placed Penrose drains in the graft tunnels are elevated, and each graft limb is pulled down through the tunnel ensuring passage of each graft limb posterior to the ureter. The common femoral, superficial femoral, and profunda branches are occluded using appropriate atraumatic vascular clamps. Occasionally, an endarterectomy of the outflow vessels may be required. A standard vascular anastomosis is then performed with monofilament suture to the bilateral femoral vessels. In cases of superficial femoral artery occlusion, the anastomosis should be performed with the hood onto the profunda femoral artery to improve patency.
Blood flow is sequentially restored to the lower extremities to minimize the potential for distal thromboemboli and “declamping hypotension.” If necessary, heparin is reversed with protamine sulfate at a dose of 1 mg/100 U heparin. The retroperitoneum is closed over the graft to aid in prevention of aortoenteric fistula. The abdomen and groin incisions are closed in a standard fashion, and the pedal pulses are checked before leaving the operating room. In cases of chronic aortic occlusion, the pedal signals may initially be poor; however, these usually improve over the course of 1 day in the ICU with patient warming. However, the lack of any popliteal Doppler signal following aortic reconstruction is a poor prognostic sign and often portends limb loss without additional infrainguinal revascularization. It is not uncommon for perfusion to continually improve over the course of the first several days, especially in patients with outflow only through the deep femoral artery (Table 1).
Special Intraoperative Considerations for Aortofemoral Bypass
Specific complications related to the aortoiliac bypass include colon/pelvic ischemia, atheroembolization, lower extremity ischemia, male sexual dysfunction, and groin wound complications. Pelvic ischemia is rare; however, it can manifest as colonic ischemia, infarction of the buttock musculature, or cauda equina/lumbar plexopathy with neurologic deficit. Risk factors for this complication include bilateral occlusion of the internal iliac arteries and end-to-end aortic reconstruction with bilateral external iliac artery occlusion. It is generally accepted that maintaining at least one internal iliac artery helps prevent this complication.
Juxtarenal aortic occlusion represents a complete thrombosis of the aorta at the level of the renal artery origin. Several important modifications of the technique are necessary. This problem is best managed by brief suprarenal clamping between the superior mesenteric and renal arteries to allow removal of the juxtarenal thrombus and safe subsequent infrarenal clamping. The approach to the suprarenal aorta remains infracolic, but may require division of the left renal vein near the vena cava to facilitate exposure. Protection of the kidneys by brief renal artery occlusion during removal of thrombus is necessary. The renal arteries can be easily controlled with vessel loops. The infrarenal aorta should never be clamped near the renal arteries until the occluding thrombotic material is removed. After suprarenal clamping, the aorta is divided several centimeters below the renal artery origin and the obstructing thrombus freed up with an endarterectomy spatula. Following removal of the juxtarenal thrombus, the aorta is clamped infrarenally, renal blood flow is restored, and a standard anastomosis is performed.
Atheromatous debris may embolize at any time during aortofemoral bypass. The most vulnerable time is when the vessels are manipulated such as during dissection or clamp application. The sequelae are dependent upon the size of the debris and the distribution of the involved vessels. Macroscopic particles may occlude the major vessels with the debris frequently lodging at the various bifurcation sites. The majority of these are amenable to removal with a thromboembolectomy catheter. In contrast, the microscopic particles lodge in smaller vessels and are not usually amenable to surgical removal, and antiplatelet and/or anticoagulation therapy is indicated.
Ischemia-reperfusion injury in aortic occlusion transpires in the setting of temporarily diminished or absent blood flow to the lower extremities, followed by the return of oxygenated blood. Reperfusion can result in tissue edema that may progress to compartment syndrome and may be severe enough to require fasciotomy. Reperfusion syndrome may lead to systemic involvement and become life threatening, as profound acidosis, hyperkalemia, myoglobinuria, renal failure, multiple organ dysfunction syndrome, and even death may occur. Therefore, prompt recognition of ischemia-reperfusion injury is essential, and fasciotomy when appropriate can limit the degree of myoglobinuria. Postoperative intubation, sedation, and critical illness often limit the value of physical examination in the determination of subsequent development of postoperative compartment syndrome. It is our practice to perform fasciotomy when severe ischemia is present for 6 hours or more preoperatively. Compartment syndrome occurs more commonly in patients with aortic embolus than aortic thrombosis, presumably due to preformed collaterals as described above in the latter condition. The treatment of rhabdomyolysis should focus on preserving renal function, and the management is supportive with aggressive fluid resuscitation, urine alkalinization, and in the setting of a restored intravascular space, the use of diuretics.
Surgical Approach to Axillobifemoral Bypass
Axillobifemoral bypass has traditionally been regarded as a second-choice alternative to elective aortofemoral reconstruction, but has significant utility in the emergent setting for acute aortic occlusion. It is the procedure of choice in the critically ill patient with aortic occlusion secondary to thrombosis of aortic aortoiliac occlusive disease. Axillobifemoral bypass is also useful in patients with multiple prior abdominal or aortic procedures in which aortofemoral bypass will be a difficult or prolonged operation that will result in delay in reestablishment of lower extremity perfusion.
TABLE 2. Axillobifemoral Bypass




