As long as the wall stress remains normal, the ventricular systolic performance (ejection fraction) will be maintained. In this setting, the cardiac output will be preserved and the patient may remain symptom-free for many years. However, this compensatory response cannot be maintained indefinitely and once the wall stress is increased, the high afterload will result in decreased ejection fraction. When the impaired systolic function of the myocardium is caused solely by increased afterload, the function will improve following aortic valve replacement. However, at some point the impaired myocardial contractility will no longer be reversible and corrective surgery will be less beneficial than in patients with reversible dysfunction due to high afterload. It is often difficult to determine when the depressed contractility is reversible and this cannot be assessed on resting imaging alone. The increased wall thickness also results in decreased compliance of the ventricular wall resulting in increased end-systolic filling pressures. Thus, dyspnea on exertion is often a symptom patients experience prior to the development of impaired ventricular function. The process of concentric hypertrophy also has another adverse consequence. The increased wall thickness may result in a reduced coronary blood flow per gram of muscle, and these blood vessels often demonstrate reduced coronary vasodilator reserve. These changes can manifest as angina even in the presence of normal epicardial coronary blood vessels.
The progression of stenosis is usually a gradual event and studies have suggested that once moderate stenosis is present, the average rate of progression is an increase in mean pressure gradient by 7 mmHg and a decrease in valve area by 0.1 cm2 per year. There is significant variability in individual cases, and the progression of stenosis may be more abrupt in patients with degenerative aortic disease compared to those with rheumatic heart disease [3]. Eventually patients manifest symptoms of angina, syncope, and/or heart failure. The appearance of these symptoms marks an important time point in the disease, as the average survival after symptom development is only 2–3 years. Symptomatic severe aortic stenosis is also associated with a substantial risk of sudden cardiac death [3, 6].
4.2.2 Physical Findings and Diagnostic Evaluation
The classic murmur of aortic stenosis is a crescendo-decrescendo systolic murmur along the left sternal border that radiates into the carotid arteries. The intensity of the murmur does not correlate with the severity of the stenosis; in fact, in severe stenosis with depressed systolic function the murmur may be almost absent. However, as the stenosis worsens, the duration of the murmur lengthens and becomes more likely to peak in mid to late systole. A delayed and reduced carotid upstroke (pulsus parvus et tardus) is also consistent with severe aortic stenosis; however the elderly patient with severe aortic stenosis may lack this finding due to stiffness of the vasculature. The first heart sound is usually normal; the second heart sound may be single due to the aortic and pulmonary components being superimposed or because the aortic component is absent or soft due to the immobility of the calcified aortic valve. The presence of a normally split second heart sound is the only physical finding that excludes the possibility of critical aortic stenosis [7].
Echocardiography is the modality of choice to evaluate for and determine the etiology of the aortic stenosis. In patients with aortic stenosis, the aortic valve is usually thickened and calcified and valve area is reduced due to the limited movement of the aortic leaflets. Using Doppler measurements, the modified Bernoulli equation, and the continuity equation, the aortic pressure gradients and aortic valve area can be calculated and the severity of the stenosis assessed as shown in Table 4.1. In patients with poor cardiac output, the calculated gradients, and aortic valve area may misestimate the true degree of aortic stenosis. In patients with true severe aortic stenosis, even though the stenosis is significant and fixed, the gradient is low because the ventricle lacks the contractile strength to generate a high-pressure gradient. In patients with low cardiac output due to primary contractile etiology, the valve area appears smaller and the aortic stenosis is worse than actual, because the ventricle cannot generate the primary force to open the thickened valve. In both situations, the low-flow state and low-pressure gradients contribute to a calculated aortic valve area that can be in the severe aortic stenosis range. In such cases, a dobutamine stress echocardiogram can help delineate the true degree of aortic stenosis. Patients with pseudostenosis will exhibit an increase in valve area and little change in transaortic pressure gradient with an increase in stroke volume, while patients with true severe stenosis will respond to the increase in stroke volume with an increase in the pressure gradient but little to no change in the aortic valve. Patients who fail to generate an increase in stroke volume in response to dobutamine infusion have a very poor prognosis with either medical or surgical therapy.
Table 4.1
Classification of the severity of aortic stenosis in adults
Indicator | Mild | Moderate | Severe |
|---|---|---|---|
Jet velocity (ms-1) | Less than 3.0 | 3.0–4.0 | Greater than 4.0 |
Mean gradient (mmHg) | Less than 25 | 25–40 | Greater than 40 |
Valve area (cm2) | Greater than 1.5 | 1.0–1.5 | Less than 1.0 |
Valve area index (cm2m-2) | Less than 0.6 |
The only indications for invasive (cardiac catheterization) assessment of aortic stenosis are finding a discrepancy between the clinical and echocardiographic findings or evaluating the presence of coronary artery disease. Due to the high concomitant frequency of aortic stenosis and coronary artery disease, coronary angiography is recommended in patients with aortic stenosis who are at risk for coronary artery disease before aortic valve replacement.
Due to the gradual progression of the disease, many patients with aortic stenosis do not recognize symptoms or may misinterpret them as normal aging and deconditioning. In these asymptomatic patients, exercise testing may elucidate patients who could benefit from earlier aortic valve surgery by identifying poor exercise capacity, an abnormal blood pressure response to exercise, or exercise-induced symptoms. However, such exercise testing should only be conducted under the close supervision of an experienced physician. Exercise testing should not be performed on symptomatic aortic stenosis patients due to the high risk of complications, nor should it be used to assess for coronary artery disease given the poor diagnostic accuracy in patients with aortic stenosis [3, 8].
4.2.3 Treatment
There is no current treatment proven to prevent or delay the development of progression of aortic stenosis. Due to the similarity of risk factors between aortic stenosis and coronary artery disease, statins have been proposed as a possible therapeutic intervention; however, to date no randomized clinical trials have supported this hypothesis.
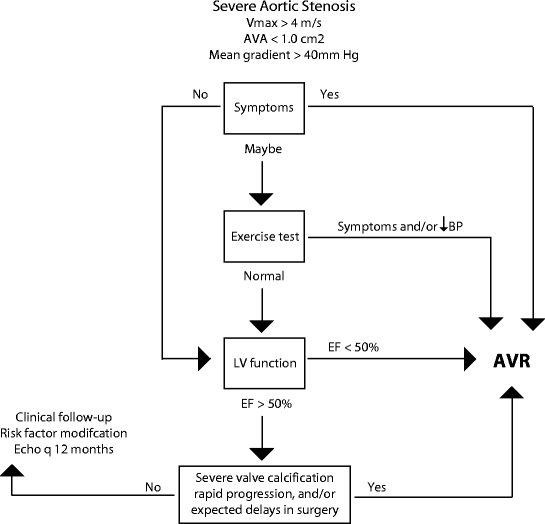
Aortic valve replacement is indicated once symptoms develop. Patients with abnormal exercise testing results or ventricular function should also be considered for valve replacement surgery. The indications for aortic valve replacement are outlined in Fig. 4.1. There has been some disagreement among cardiologists regarding the indication of aortic valve replacement in asymptomatic patients with severe aortic stenosis due to the finding that, without surgery, less than 50% of these patients will continue to be symptom-free at 5 years. There was also concern that myocardial fibrosis or irreversible ventricular dysfunction may occur during a prolonged asymptomatic phase. These concerns have not yet been substantiated; therefore the current recommendation is that the risk of aortic valve replacement is higher than the potential benefit in these patients who are truly asymptomatic with severe aortic stenosis. Patients should be followed closely with serial examinations and careful attention noted to development of symptoms or change in ventricular function. However, future recommendations for this group of patients may change as newer, safer, and less-invasive options of aortic valve replacement become available. Catheter-based placement of bioprosthetic valves is an exciting new therapy that is currently being used in experimental settings for patients with severe aortic stenosis who are not candidates or who are very high-risk candidates for conventional surgical aortic valve replacements.