Chapter 60 Acquired Heart Disease
Coronary Insufficiency
Coronary Artery Anatomy and Physiology
Anatomic Considerations
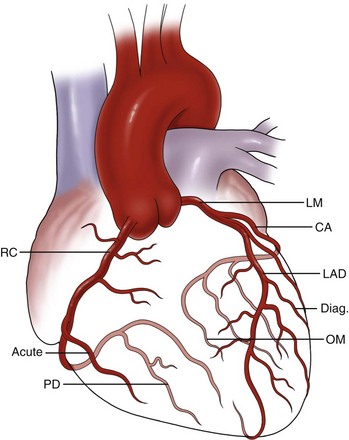
The coronary arteries are the predominant blood supply conduits to the heart. The coronary arteries arise from the sinuses of Valsalva, which are elastic saccular bulges of the aortic root. The coronary arteries are the first arterial branches of the aorta and usually two are present. They are designated as right and left according to the embryologic chamber that they predominantly supply. The left coronary artery (LCA) arises from the left coronary sinus, which is located posteromedially, whereas the right coronary artery (RCA) arises from the right coronary sinus, which is located anteromedially. The LCA, also called the left main coronary artery, averages approximately 2 to 3 cm in length and courses in a left posterolateral direction, winding behind the main pulmonary artery trunk and then splitting into the left anterior descending (LAD) and left circumflex arteries. The LAD courses in an anterolateral direction to the left of the pulmonary trunk and runs anteriorly over the interventricular septum. The diagonal branches of the LAD supply the anterolateral wall of the left ventricle. The LAD is considered the most important surgical vessel because it supplies more than 50% of the left ventricular mass and most of the interventricular septum. The LAD has several septal perforating branches that supply the interventricular septum from the anterior aspect. The LAD extends over the interventricular septum up to the apex of the heart, where it may form an anastomosis with the posterior descending artery (PDA), which is typically a branch of the right coronary system (Fig. 60-1).
The RCA supplies most of the right ventricle, as well as the posterior part of the left ventricle. The RCA emerges from its ostium in the right coronary sinus, passes deep in the right AV groove, and then proceeds over the anterior surface of the heart. At the superior end of the acute margin of the heart, the RCA turns posteriorly toward the crux and usually bifurcates into the PDA over the posterior interventricular sulcus and right posterolateral artery. The RCA also supplies multiple right ventricular branches (acute marginal branches). Occasionally, the PDA arises from both the RCA and LCA and the circulation is considered to be codominant. The AV node artery arises from the RCA in approximately 90% of patients. The sinoatrial node artery arises from the proximal RCA in 50% of patients. Other prominent branches arising from the RCA include the acute marginal artery and anterior ventricular branches. Although the source of the PDA is often used clinically to define dominance of circulation in the heart, anatomists define it according to where the sinoatrial node artery arises. Table 60-1 summarizes the hierarchy of the coronary artery anatomy.
Table 60-1 Anatomic Architecture of Coronary Arteries
| NAMED VESSELS | BRANCHES |
|---|---|
| Left main coronary artery | |
| Left anterior descending | |
| Circumflex coronary artery | |
| Right coronary artery |
Physiology and Regulation of Coronary Blood Flow
Because the myocardium has a high rate of energy use, normal coronary blood flow averages 225 mL/min (0.7 to 0.9 mL/g of myocardium/min) and delivers 0.1 mL/g/min of oxygen to the myocardium. Under normal conditions, more than 75% of the delivered oxygen is extracted in the coronary capillary bed, so any additional oxygen demand can only be met by increasing the flow rate. This highlights the importance of unobstructed coronary blood flow for proper myocardial function. Box 60-1 summarizes the unique features of coronary blood flow.
History of Coronary Artery Bypass Surgery
The development of the heart-lung machine and demonstration of successful clinical use by Dr. John Heysham Gibbon in the 1950s and the advancement of cardioplegia techniques in later years by Dr. Gerald Buckberg allowed surgeons to perform coronary anastomosis on an arrested (nonbeating) heart with a relatively bloodless field, thus increasing the safety and accuracy of the coronary bypass. In the 1990s, the advent of devices that could atraumatically stabilize the heart provided another pathway for the development of off-pump techniques of myocardial revascularization. Today, an armamentarium of techniques, ranging from conventional on-pump CABG to minimally invasive robotic and percutaneous approaches, is available to manage coronary artery disease. Table 60-2 summarizes the timeline of major historical events in the development of surgery for myocardial revascularization.
Table 60-2 Evolution of Surgical Coronary Artery Interventions: Timeline
| 1950 | A. Vineberg | Direct implantation of mammary artery into myocardium |
| 1953 | J. H. Gibbon | First successful use of cardiopulmonary bypass machine |
| 1962 | F. M. Sones | Successful cine-angiography |
| 1964 | M. E. DeBakey | First successful coronary artery bypass grafting |
| 1964 | T. Sondergaard | Introduced routine use of cardioplegia for myocardial protection |
| 1964 | D. A. Cooley | Routine use of normothermic arrest for all cardiac cases |
| 1968 | R. Favoloro | First large series demonstrating success of CABG |
| 1973 | V. Subramanian | Beating heart coronary artery bypass graft |
| 1979 | G. Buckberg | Introduced the use of blood cardioplegia as preferred method for to arrested myocardial protection |
Atherosclerotic Coronary Artery Disease
Epidemiologic evidence suggests that coronary artery atherosclerosis is closely linked to the metabolism of lipids, specifically LDLc. The development of lipid-lowering drugs has resulted in a significant reduction in mortality. In one observational study of patients who received statin therapy and were known to have coronary artery disease (CAD), statin treatment was associated with improved survival in all age groups.1 The greatest survival benefit was found in those patients in the highest quartile of plasma levels of high-sensitivity C-reactive protein (hs-CRP), a biomarker of inflammation and CAD.2 Animal and human studies have demonstrated that statin therapy also modifies the lipid composition within plaques by lowering the amount of LDLc and stabilizing the plaque through various mechanisms, including reduction in macrophage accumulation, collagen degradation, reduction in smooth muscle cell protease expression, and decrease in tissue factor expression.
Pathogenesis
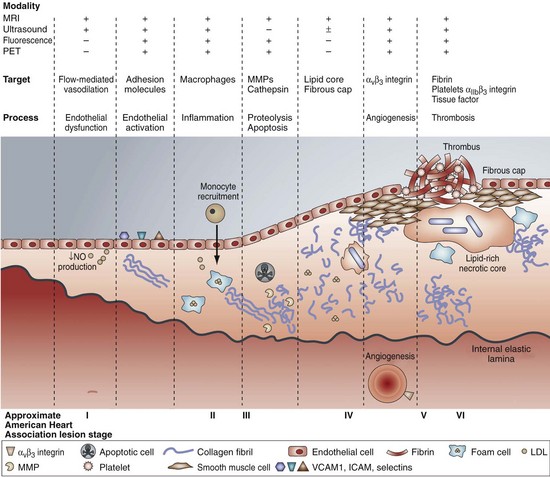
Thinner fibrous caps are at a higher risk for rupture, probably because of an imbalance between the synthesis and degradation of the extracellular matrix in the fibrous cap that results in an overall decrease in the collagen and matrix components (Fig. 60-2). Increased matrix breakdown caused by matrix degradation by an inflammatory cell-mediated metalloproteinase or reduced production of extracellular matrix results in thinner fibrous caps. Not all plaque ruptures are symptomatic; whether they are is dependent on the thrombogenicity of the plaque’s components. Tissue factor within the lipid core of the plaque, secreted by activated macrophages, is one of the most potent thrombogenic stimuli. Rupture of a vulnerable plaque may be spontaneous or caused by extreme physical activity, severe emotional distress, exposure to drugs, cold exposure, or acute infection.
Clinical Manifestations and Diagnosis of Coronary Artery Disease
Clinical Presentation
The prehospital mortality rate for an acute MI (AMI) is approximately 50%. Of those patients who reach the hospital, another 25% die during the hospital stay and another 25% die in the first year afterward.3,4 Mechanical complications of MI include acute ventricular septal defect (VSD), papillary muscle rupture, and free ventricular rupture. They usually occur approximately 7 to 10 days after the initial MI.
Physical Examination
Some clinical findings are generic and are related to the systemic manifestations of atherosclerosis. Eye examination may reveal a copper wire sign, retinal hematoma or thrombosis secondary to vascular occlusive disease, and hypertension. Corneal arcus and xanthelasma are features noticed in cases of hypercholesterolemia. Other clinical manifestations are caused by sequelae of CAD, as noted in Box 60-2.
Box 60-2 Sequelae of Coronary Artery Disease: Clinical Manifestations
Diagnostic Testing
Risk Stratification and Further Testing
An exercise stress ECG is helpful in unmasking underlying CAD and is a more reliable screening test than a resting ECG in patients older than 40 years. The Bruce protocol is the most commonly used standardized treadmill exercise protocol. The protocol involves five 3-minute bouts of treadmill exercise, each designed to elicit greater myocardial oxygen demand than the last, to determine the patient’s ischemic threshold. A typical protocol requires the patient to expend about 12 metabolic equivalents (METs) of energy to ensure a complete test. A positive exercise ECG may show progressive flattening of the ST segment or ST-segment depression as exercise progresses. During the recovery phase, ST depression may persist, with downsloping segments and T wave inversion. Additional findings associated with an adverse prognosis and the presence of multivessel occlusive disease include a duration of symptom-limited exercise of less than 6 METs, the failure of systolic blood pressure to increase to more than 120 mm Hg, and the appearance of ventricular arrhythmias. For detection of CAD, the sensitivity and specificity of an exercise ECG approach 70% and 80%, respectively (Box 60-3).
BOX 60-3 Stress Tests to Identify Coronary Artery Disease
Multidetector Computed Tomography
Multidetector computed tomography (MDCT), one of the most recent imaging modalities, allows imaging of the coronary arteries, especially of coronary artery bypass grafts. Studies have indicated that the sensitivity and specificity MDCT approach or exceed those of other noninvasive methods of visualizing the coronary artery anatomy.5 MDCT is especially useful for imaging proximal CAD and coronary artery bypass grafts. More recent technology improves on conventional MDCT by adding more arrays to the imaging process; 128-slice MDCT arrays are currently available. These scanners can acquire myocardial images within 1 second while exposing the patient to less radiation than traditional scanners. Although it is still preferable that patients have relatively low heart rates during imaging (to reduce artifact), the technology has significantly advanced and produces images on par with those generated by the gold standard, conventional angiography.6
Cardiac Catheterization and Intervention
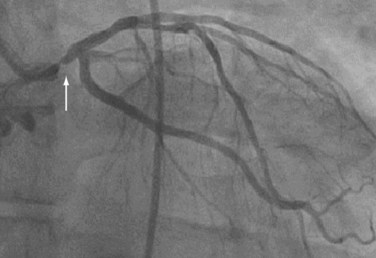
Cardiac catheterization is commonly performed by inserting a short, self-sealing vascular sheath into either femoral artery. Vascular access may also be obtained via a brachial or radial artery. Angiography is done by using hollow preshaped catheters (5 or 6 Fr), which are placed under fluoroscopic guidance retrograde through the aorta into the ostia of the coronary arteries and coronary bypass grafts. A solution of radiographic contrast material is injected through the catheter to opacify the lumen. Images are recorded in rapid succession onto film or in a digital format. The surgeon typically uses the coronary angiography images to determine the number and location of coronary targets where bypass anastomoses are to be constructed (Figs. 60-3, 60-4, and 60-5).
Indications for Coronary Artery Revascularization
Box 60-4 summarizes the indications for myocardial revascularization. The first four indications are managed preferably by PCI, whereas indications 5 through 7 are managed preferably by surgical revascularization. The last two indications constitute surgical emergency. Although this stratification is broad and provides a bird’s eye view of the management approach, each patient should be risk-stratified before an appropriate strategy is initiated. When possible, proper risk stratification is absolutely essential to determine the balance of risks and benefits of medical management, PCI, and CABG.
BOX 60-4 Indications for Coronary Artery Revascularization
Chronic Stable Angina
Cardiovascular risk reduction strategy is essential to treating patients with chronic stable angina. In the 2007 focused update of the 2002 American Heart Association (AHA)/American College of Cardiology (ACC) guidelines7 for managing chronic stable angina, cardiovascular risk reduction strategies included smoking cessation, blood pressure control, lipid-lowering regimens, physical activity, antiplatelet agents, angiotensin-converting enzyme (ACE) inhibitors, weight control, diabetes management, and influenza vaccination. Such risk reduction strategies should be used for all patients, irrespective of the type of intervention planned on the coronary artery.
Percutaneous Coronary Intervention Versus Medical Management
In the 1980s, PCI was introduced as an alternative to CABG. Although the short-term symptomatic success rate for PCI approaches 85% to 90%, the usefulness of PCI remains controversial for patients with angina whose symptoms are adequately controlled with medical therapy.8,9 The main results of the VA Clinical Outcomes Utilizing Revascularization and Aggressive druG Evaluation (COURAGE) trial revealed no significant differences in the primary end point of all-cause mortality or nonfatal MI, or in the major secondary end points, during a median 4.6-year follow-up period in patients with stable CAD who were randomly assigned to receive optimal medical therapy (OMT), with or without PCI.10
Coronary Artery Bypass Grafting Versus Percutaneous Coronary Intervention
Pre–Stent Era
One of the first large-scale, prospective, randomized studies of PCI and CABG was the Bypass Angioplasty Revascularization Investigation (BARI) trial reported in 1996.11 Patients with multivessel disease were randomly assigned to CABG or PCI and followed up for a mean of 5.4 years. In the short term, the incidence of MI was higher in the CABG group (4.6% versus 2.1%), but stroke rates were similar (0.8% versus 0.2%, CABG versus PCI). Five years after treatment, the survival rate was 89.3% for the CABG cohort and 86.3% for the PCI cohort (P = .19). Of the PCI patients, however, 54% required additional revascularization procedures, whereas only 8% of the CABG patients required repeat revascularization. Thus, although PCI did not compromise the 5-year survival rate in patients with multivessel disease, subsequent revascularization, including CABG, was required more often. Among the diabetic patients, the 5-year survival rate for the CABG patients was markedly greater (80.6% versus 65.5%).
Bare Metal Stent Era
In the 1990s, coronary stents were introduced to address the problematic occurrence of restenosis after PCI. Six randomized trials have compared PCI with stenting and CABG.12–14 Except for the Angina With Extremely Serious Operative Mortality Evaluation (AWESOME) trial, these trials enrolled patients who were relatively low risk and had no serious comorbidities, normal ventricular function, and mostly two-vessel CAD that was amenable to both PCI and CABG. All these trials showed similar survival rates but there were higher revascularization rates in patients with bare metal stents. A meta-analysis of four randomized trials has shown that PCI with stenting is associated with a long-term safety profile similar to that of CABG. However, as a result of persistently lower repeat revascularization rates in the CABG patients, overall major adverse cardiac and cerebrovascular event rates were significantly lower in the CABG group at 5 years.15 Although these randomized studies are often used to demonstrate survival equivalence of CABG and PCI in patients with multivessel CAD, the studies were underpowered, the patients were low-risk, and the follow-up was too short. In contradistinction, a large New York State registry study has demonstrated that patients with double- or triple-vessel disease derive a greater survival benefit from CABG than from PCI with stenting.16 Analysis of this large database of more than 50,000 patients found that during the 3-year follow-up, repeat revascularization was 11 times higher in the percutaneous transluminal coronary angioplasty (PTCA) group (37% versus 3.3% CABG). Furthermore, 3-year mortality was significantly higher in the PTCA group.
Drug-Eluting Stent Era
The proponents of drug-eluting stent (DES) implantation claim that improved technology has made the results of randomized controlled trials (RCTs) favoring CABG obsolete. However, in patients with multivessel disease, PCI with DES can produce survival rates equivalent to those associated with CABG only if the reduction in restenosis rate translates into reduced mortality. Additionally, no mortality benefit of DES compared with bare metal stents has been demonstrated; in a meta-analysis of 11 RCTs of PCI with DES versus bare metal stents, none of the trials found a mortality benefit for DES.17
Acute Coronary Syndrome
Unstable Angina and Non–ST-Segment Elevation Myocardial Infarction
Patients who present with unstable angina (UA) or NSTEMI may have associated symptoms that confer a high short-term risk of death that necessitates invasive intervention. Two treatment pathways are used for treating UA-NSTEMI patients: the early invasive strategy and an initial conservative strategy. Patients treated with an invasive strategy generally undergo coronary angiography within 4 to 24 hours of admission.2 Estimating the risk of an adverse outcome is paramount for determining which strategy is best for an individual patient. High-risk patients who benefit from invasive therapy include those with recurrent angina, ischemia at rest, low-level activity despite intensive medical therapy, elevated levels of cardiac biomarkers (troponins), new ST-segment depression, signs or symptoms of congestive heart failure or of new or worsening mitral regurgitation, findings from noninvasive testing that suggest high risk, hemodynamic instability, sustained ventricular tachycardia, PCI within the previous 6 months, prior CABG, and reduced left ventricular function (<40%).
According to the AHA/ACC/American Association for Thoracic Surgery (AATS) guidelines, UA-NSTEMI and features associated with high short-term risk of death or nonfatal MI indicate revascularization of the presumed culprit artery. These indications are similar to those for coronary revascularization in patients with chronic stable angina.18
ST-Segment Elevation Myocardial Infarction–Acute Myocardial Infarction
Percutaneous Coronary Intervention Versus Medical Management for Acute Myocardial Infarction
In general, PCI has a survival advantage over thrombolytics as an initial treatment for STEMI-AMI, and the use of delayed PCI as an adjunct to therapy, including therapy with thrombolytics, does not affect survival. In the Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO) IIb trial,19 the 30-day rate of the composite end point of death, nonfatal MI, and nonfatal disabling stroke was 9.6% for PCI patients and 13.7% for recipients of thrombolytics.
Prospective observational data collected from the Second National Registry of Myocardial Infarction between June 1994 and March 1998 included a cohort of 27,080 consecutive patients with AMI associated with ST-segment elevation or left bundle branch block. These patients were all treated with primary angioplasty; the study revealed that the adjusted odds of mortality were significantly higher (62% versus 41%) for patients with door to balloon times longer than 2 hours. The longer the door to balloon time, the higher the mortality risk, emphasizing that door to balloon time has a significant impact on the outcomes for patients with AMI.20
On the basis of this evidence, PCI facilities have been required to establish a target door to balloon time of no longer than 90 minutes. Depending on the available facilities in a particular region, it is the responsibility of emergency medical services (EMS) personnel to determine whether that goal can be achieved by transferring the patient to a PCI-capable facility. If this cannot be accomplished, a medical management strategy should be considered, with the goal being a door to needle time of 30 minutes or less.21
Role of Coronary Artery Bypass Grafting
Although an increasing number of patients undergo catheterization early after AMI, the initial treatment is directed by the interventionalist, which has significantly diminished the role of emergency CABG. In general, patients who undergo CABG early after AMI are sicker and efforts to improve myocardial function are typically refractory to medical therapy. These patients typically have a higher incidence of comorbidities and are more likely to require intra-aortic balloon pump (IABP) insertion. The optimal timing of CABG after AMI is not well established. A review of California Discharge Data has identified 9476 patients who were hospitalized for AMI and subsequently underwent CABG. Of these, 4676 (49%) were in the early CABG group and 4800 (51%) were in the late CABG group. The mortality rate was highest among patients who underwent CABG on day 0 (8.2%) and declined to a nadir of 3.0% among patients who underwent CABG on day 3. The mean time to CABG was 3.2 days. Early CABG was an independent predictor of mortality, suggesting that CABG may best be deferred for 3 or more days after admission for AMI in nonurgent cases.22
The SHOCK (Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock) trial has shown the survival advantage of emergency revascularization versus initial medical stabilization in patients in whom cardiogenic shock developed after AMI. A subanalysis that compared the effects of PCI and CABG on 30-day and 1-year survival showed that survival rates were similar at both time points. Among SHOCK trial patients randomly assigned to undergo emergency revascularization, those treated with CABG had a greater prevalence of diabetes and worse coronary disease than those treated with PCI. However, survival rates were similar.23
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree