Chapter 86
Acquired Arteriovenous Fistulae
Wei Zhou
Based on a chapter in the seventh edition by Glenn C. Hunter
An arteriovenous fistula (AVF) is an abnormal connection between an artery and a vein. The earliest description of an arteriovenous fistula is credited to William Hunter, who in 1761 reported two patients with brachial AVFs following attempted phlebotomy. He also described the adaptive changes that accompany a communication between the brachial artery and vein: enlargement of the artery proximal to the AVF and a weak pulse below the lesion; veins in the vicinity of the AVF also became enlarged and were associated with a hissing noise and tremulous jarring motion that faded a short distance from the fistula.1,2 What Hunter described were the most common physiologic effects of an AVF on involved arteries and the most appreciated sign of AVFs on physical examination, a thrill.
AVFs remained uncommon until the 19th century, when the introduction of high-speed projectiles changed the magnitude and complexity of military injuries. Successive military conflicts and the increase in penetrating trauma among civilians resulted in a greater number of vascular injuries and, as a consequence, AVFs. However, prompt evacuation of victims from the battlefield, more effective resuscitation, and direct arterial and venous repair of injuries, begun during the Korean War and refined during the Vietnam and Iraq conflicts, have significantly reduced the number of traumatic AVFs encountered despite the increase in the incidence of penetrating vascular injuries.3–7
The focus of this chapter is acquired AVFs. Chapters 69 to 72 cover vascular malformations including congenital AVFs.
Etiology and Incidence
The primary etiologies of acquired AVFs are traumatic injuries and iatrogenic injuries. A small number occur spontaneously, usually from erosion of an aneurysm into an adjacent vein.
Traumatic Injuries
Stab wounds, the most likely civilian trauma to result in an AVF, accounted for 63% of the 202 traumatic AVFs reported by Robb et al; gunshot wounds (GSWs) accounted for 26%, and blunt trauma for 1% of AVFs in the remaining patients.10 Because shotguns disperse a few to hundreds of pellets, multiple puncture wounds resulting in AVFs in many locations can occur. Although extremity and abdominal vascular injuries occur with almost equal frequency (40% versus 32%) in the civilian population,9 approximately 22% of AVFs are located in the upper extremity and 20% in the lower extremity, whereas intrathoracic and intraabdominal AVFs are encountered in only 4%.10 Similarly, extremity AVFs following military trauma are much more common than abdominal and thoracic AVFs.11
Iatrogenic Injuries
The increase in numbers of interventional procedures (both vascular and nonvascular) performed over the past 10 years has resulted in an increase in the complication of iatrogenic AVFs. The majority of iatrogenic AVFs associated with percutaneous interventions are small, with shunt volumes ranging from 60 to 510 mL/min, well below that seen with hemodialysis fistulae. These relatively low flow rates presumably account for the infrequent occurrence of detrimental cardiac effects seen with larger fistulae, but they do not predict the likelihood of fistula closure.12 The natural history of stable iatrogenic pseudoaneurysms and AVFs is relatively benign, with 38% to 81% of fistulae expected to close spontaneously.12,13,20
Spontaneously Acquired AVF
Inflammatory processes and pathologic changes to the arterial wall have contributed to the development of spontaneously acquired AVFs. First described by Syme in 1831, spontaneous rupture of an aortic or iliac aneurysm or erosion of an inflammatory or mycotic aneurysm into a contiguous vein, such as the inferior vena cava (IVC) (Fig. 86-1), iliac vein (Fig. 86-2), or left renal vein (Fig. 86-3), can result in a spontaneously acquired AVF.66–71 A few isolated cases have also been reported in association with inflammatory, syphilitic, or human immunodeficiency virus arteritis, as well as with Marfan’s and Ehlers-Danlos syndromes.67,75 Patients with spontaneously acquired AVFs are predominantly male, with an average age of 65 years.69
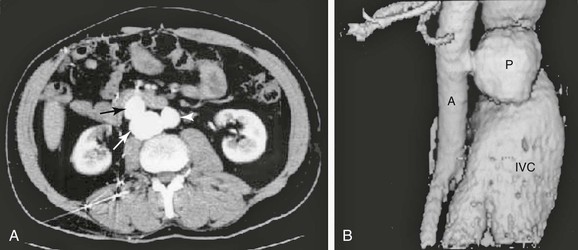
Figure 86-1 A, Axial CT scan demonstrating the aorta (arrowhead), pseudoaneurysm (white arrow), and inferior vena cava (black arrow). B, Three-dimensional reconstruction of the CT scan of a traumatic aortocaval fistula demonstrating a pseudoaneurysm (P), enlarged inferior vena cava (IVC), and aorta (A).
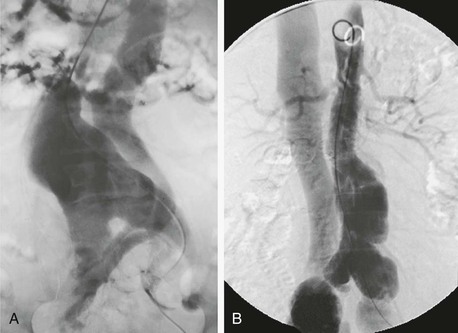
Figure 86-2 A, Aortogram of a patient with an aortic aneurysm and spontaneous aortocaval fistula treated surgically. B, Aortogram demonstrating an aortic aneurysm and bilateral iliac aneurysms associated with an arteriovenous fistula between the right common iliac artery and vein treated with an aortic endograft.
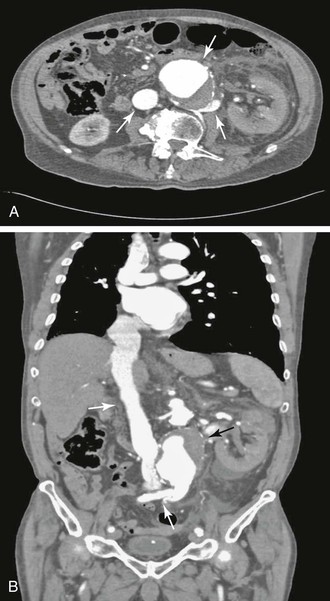
Figure 86-3 A, Axial CT scan demonstrating an aortorenal arteriovenous fistula. Contrast material is present in the abdominal aortic aneurysm (top arrow), inferior vena cava (left arrow), and retroaortic left renal and lumbar veins (right arrow). B, Sagittal reconstruction of the CT scan in A showing contrast material in the aneurysm (black arrow), inferior vena cava (top white arrow), and retroaortic left renal and lumbar veins (bottom white arrow).
Incidence of AVFs
Carotid Artery
Carotid artery injury constitutes 5% to 11% of all arterial injuries and is most often the result of high-velocity penetrating trauma and stab wounds, therapeutic or diagnostic catheterizations in the neck, craniocervical fractures, or blunt trauma to the neck. The incidence of carotid AVFs from penetrating trauma ranges from 4% to 27%. The communication is usually between the carotid artery and the internal jugular vein or its tributaries.7,10,11,18,19,21,22
Subclavian and Axillary Arteries
Approximately 3% of all neck and chest trauma is associated with injuries to the subclavian artery or vein.23 The subclavian vein is involved in 44% of cases and the artery in 39%; in the remaining 17% of patients, both vessels are injured. As previously noted, most of these vascular injuries are due to penetrating injuries; fractures of the clavicle and first rib rarely cause vascular injury.23,24 In a series of 57 subclavian injuries reported by du Toit et al, 23% resulted in AVFs.26 In contrast, AVFs are less commonly associated with penetrating axillary artery injuries than with subclavian trauma, with only one AVF identified in a review of 85 penetrating axillary artery injuries.27
Central venous catheterization and therapeutic catheter manipulations to treat dialysis access–related complications may also be complicated by subclavian and axillary AVFs. Inadvertent puncture of the subclavian or carotid arteries occurs with sufficient frequency (up to 8.4%) that one should consider the possibility of this complication if a pulsatile mass or bruit becomes evident at the site of catheterization in these areas.18,19 It is estimated that the incidence of AVF following subclavian vein catheterization is 0.58%.25
Vertebral Artery
Vertebral artery AVFs are characterized by a communication between the vertebral artery and the internal jugular and/or vertebral veins. The incidence of vertebral artery injury due to penetrating trauma ranges from 0.7% to 7.4%, depending on the mechanism of injury (gunshot versus stab wound) and the indications for angiography, which demonstrates these injuries.8,28 Blunt trauma from cervical spine fractures, chiropractic manipulation, or closed head injury accounts for a small number of cases.28 The vertebral artery can also be injured during operative procedures on the cervical spine and catheterization of the jugular vein. In a series of vertebral AVFs reported by Vinchon et al, 56% were the result of trauma; of these, 64% (18 of 28) were due to iatrogenic injury.28,29
Brachial and Forearm Arteries
Upper extremity vascular injuries accounted for 25% of all vascular injuries in a report by Clouse et al7 Brachial and forearm arteries were involved in 22% of cases and were transected in 23% of cases reported by Fox et al, with no mention of AVFs involving these vessels.6 In a series of civilian AVFs due to stab wounds, 22% of which were in the upper extremity, 12% of patients had AVFs involving forearm vessels.10 Other potential causes of AVFs in these locations include diagnostic and therapeutic cardiac and vascular catheter manipulations to treat coronary, visceral, and subclavian stenotic lesions and the placement of percutaneous intravenous central catheters.30–32
Femoral Artery
The common femoral artery is the preferred access site in about 85% to 90% of percutaneous cardiovascular interventions and is also the most common site of iatrogenic AVFs. In addition to the common femoral artery, the superficial femoral artery and profunda femoris artery are frequently involved. The incidence of iatrogenic AVFs diagnosed by physical examination ranges from 0.006% to 0.86% following cardiac catheterization, and femoral AVFs are slightly more frequent after interventional rather than diagnostic procedures. A higher incidence of AVFs (2.8%) has been reported when duplex scanning is used to evaluate patients after cardiac procedures.12,13 The factors predisposing to iatrogenic femoral AVFs are listed in Box 86-1.
Penetrating injuries due to GSWs, stab wounds, or fragment injuries from improvised explosive devices or fractures of the long bones can also lead to femoral AVFs.6,7,12,13,33 The incidence of AVFs as a result of such injuries varies considerably between the military and civilian populations. In a review of 558 AVFs and pseudoaneurysms due to military injuries reported by Rich et al, 28% (74 of 262) of the AVFs involved the superficial femoral or profunda femoris arteries,11 whereas in a civilian series of AVFs predominantly due to stab wounds, the femoral artery was involved in 12% of cases.10 Sporadic cases have also been reported of spontaneous AVFs involving the superficial femoral artery and vein.75 With increased use of endovascular techniques for venous interventions, AVFs following endovenous ablation for varicose veins have also been reported.140
Popliteal Artery
Popliteal AVFs have a similar etiology to femoral AVFs. The number of iatrogenic popliteal AVFs can be expected to increase, owing to use of the popliteal vein for the diagnosis and endovascular treatment of deep venous thrombosis. Blunt trauma associated with comminuted femur and proximal tibia fractures and orthopedic procedures on the knee may also be complicated by AVFs.35,36 Vascular injuries associated with total knee arthroplasty and arthroscopic procedures on the knee occur in approximately 0.08% of patients undergoing these procedures.36 AVFs of the popliteal artery account for approximately 16% of AVFs reported in the Vietnam registry and 5% to 14% of civilian AVFs.10,11,34
Tibial and Peroneal Arteries
AVFs of tibial and peroneal arteries are a recognized complication of comminuted leg fractures or fragment injuries of the lower extremity and of balloon catheter thrombectomy of these vessels.37,38 Approximately 24% of the AVFs reported in the Vietnam registry involved the tibial and peroneal arteries and largely resulted from fragment wounds.11 The use of technically complicated techniques and aggressive interventional approaches can lead to higher risk of AVFs in these arterial beds.14–17
Aortoiliac
Penetrating trauma due to GSWs, fragments, or stab wounds accounts for 10% to 20% of aortocaval fistulae (see Fig. 86-1). Less commonly, iatrogenic injury during arterial and cardiac catheterization or lumbar disk surgery and, rarely, blunt trauma have been implicated as causative factors in aortic AVFs.39–42 An additional cause is spontaneous AVF. Rupture or erosion of an abdominal aortic aneurysm (AAA) into the IVC accounts for 80% to 90% of patients with spontaneously acquired AVF. The incidence of spontaneously acquired AVF ranges from 0.2% to 1.3% of patients with AAAs and 3% to 4% of those with ruptured aneurysms.66–70
Iliac AVFs are uncommon (0.4% to 1.4% of AVFs) and are usually the result of penetrating trauma or lumbar disk surgery.10,11,43 In the case of lumbar disk surgery, the integrity of the anterior longitudinal ligament is the major barrier between the disk and the aorta, vena cava, and iliac vessels. Anatomic anomalies in these locations may contribute to the occurrence of vascular injuries during orthopedic procedures. Studies have shown that the aortic bifurcation is at the center of the L4-L5 disk space in approximately 18% of patients.65 The left common iliac artery and vein are the most common sites of injury with L4-L5 diskectomy, injury to the artery occurring in 77% of cases and injury to the vein occurring in 31% of cases.62 However, injuries to the right common iliac artery, IVC, aorta, and iliac veins have all been reported. Although acute arterial or venous bleeding from the operative site is the usual presentation of vessel injury during lumbar disk procedures, there have been a number of case reports of acute AVFs following lumbar diskectomy.63,64
Renal
Renal AVFs occur most commonly after renal biopsies. Factors predisposing to biopsy-related renal AVFs include use of a large-bore needle, lack of radiologic guidance when performing the biopsy, medullary penetration, and the presence of atherosclerotic vessels.44,45 Other causes of renal AVFs include placement of percutaneous nephrostomy tubes for ureteric obstruction, laser lithotripsy, and mass ligation of the renal pedicle.44–47 Renal artery aneurysms may also erode into the renal vein or one of its tributaries resulting in renal AVFs.70,71 Renal AVFs are discussed further in Chapter 148.
The incidence of biopsy-associated AVF depends on the timing of the investigative testing. Prospective angiographic studies performed immediately after biopsy have demonstrated an AVF incidence of 9% to 11%; this increased to 12% to 18% if angiography was performed 1 to 6 months after biopsy. When angiography was performed between 6 weeks and 4 years after the initial diagnosis of an AVF, 33% to 90% of the AVFs had resolved spontaneously. This is not entirely unexpected because the communication between the vein and artery in patients with procedure-related renal AVFs is usually small.44 Isolated renal AVFs due to blunt and penetrating trauma are uncommon.
Injuries involving the splanchnic arteries and the portal vein or its tributaries can result in visceral and arterioportal AVFs. These communications may involve the splenic, hepatic, and superior and inferior mesenteric arteries or their branches48–61 and may communicate directly with the portal vein, with its tributaries, or with tributaries of the inferior vena cava. Tomczak et al reported that 37% of arterioportal AVFs resulted from penetrating trauma and 27% from interventional procedures, with the remainder from congenital and other causes.48
Splenic AVFs may be due to blunt or penetrating intra-abdominal trauma, mass ligation of the splenic pedicle, iatrogenic injury during splenoportography, and in rare instances, erosion of a pancreatic pseudocyst into the splenic vessels.49–53 Rupture of splenic artery aneurysms into an adjacent major vein is sometimes the first manifestation of these lesions.57 In fact, 44% of splenic AVFs are due to rupture of a preexisting aneurysm into the splenic vein or one of its tributaries, especially in patients with noncalcified aneurysms.51 Hepatic AVFs are usually the result of vehicular or penetrating trauma but may occur after percutaneous needle biopsy and transhepatic diagnostic catheterizations or biliary drainage procedures.53–59 Hepatic AVFs occur in 5.4% of patients after percutaneous needle biopsy evaluated with celiac angiography and may be either intrahepatic or extrahepatic.53–59 Hepatic aneurysms and carcinomas are also known to erode into an adjacent artery or vein, resulting in an AVF.55,73,74 AVFs of the superior mesenteric artery are rare and are usually the result of penetrating trauma or iatrogenic injuries to the artery or its branches.60 AVFs may also occur after mass ligation of the vascular pedicle during gastric and small and large bowel resection.61
Pathophysiology
A clear understanding of the hemodynamic and structural changes induced by AVFs is critical in recognizing the local and systemic manifestations and initiating appropriate treatments for AVFs. The natural history of a communication between an artery and its contiguous vein is determined by the diameters of the artery and vein, the size and location of the fistula, the adequacy of the collateral circulation, and the competence of the valves in the distal veins. Although some fistulae reduce in size, or even close spontaneously, a large number of fistulae become more prominent over time with larger communications as a result of degenerative changes in the arterial wall.76,77
Fistula Size and Flow
The relationship between the size of the fistula and direction of flow has been elegantly demonstrated by Holman and Taylor, who found that the direction of flow in the distal artery is maintained when the cross-sectional area of the fistula is equal to or less than 1.5 times the diameter of the inflow artery, but the flow is diminished or the direction reversed when the fistulous opening exceeds the diameter of the inflow artery by more than threefold.78 Flow in the artery proximal to the fistula increases up to fivefold if the fistula is equal to or less than 1.5 times the arterial diameter and eightfold if the opening is more than three times larger than the inflow artery.79 As the flow through an acute fistula increases, the diameter of only the proximal vein increases, with no change in antegrade flow in the distal vein.76,77
Chronic Changes
In a chronic AVF, the proximal artery enlarges and becomes elongated and tortuous. Degenerative changes characterized by atrophy of the elastic lamellae and smooth muscle cell layers and deposition of lipid and calcium result in features resembling atherosclerosis. Thinning of the arterial wall and, ultimately, aneurysmal dilatation occur with long-standing fistulae.80–82 The proximal vein also increases in diameter and becomes tortuous with pulsatile flow, and the wall of the vein becomes thickened or “arterialized” as a result of the increase in hemodynamic shear stress. These structural changes, which first become evident at approximately 2 months and are established by 15 months, appear to be reversible if the AVF is repaired within 2 years of its occurrence.80–82
Although the direction of flow in the distal artery in a chronic AVF may be either antegrade or retrograde, depending on the size of the fistula, it is usually reversed with large defects owing to an increase in collateral flow distal to the fistula. The high venous pressure at the site of the fistula also results in dilatation and valvular incompetence of the distal vein. Dilatation of venous collaterals manifests as varicose veins, pigmentation, and ultimately, ulceration of the involved extremity. AVFs are associated with pseudoaneurysms in a variable number of cases, and enlargement of the pseudoaneurysm and AVF contributes to the size of the pulsatile mass that is characteristic of AVFs.
Cardiac Effects
In patients with large AVFs, the increase in venous return results in cardiomegaly, which may develop acutely within weeks or months of the injury or may progressively increase in severity over a period of years. The ability of the myocardium to compensate for the ever-increasing preload is more likely in young, otherwise healthy individuals. Ultimately, if left untreated, cardiac decompensation ensues, and the patient manifests the symptoms and signs of high-output congestive heart failure (CHF). The increase in cardiac size is accompanied by a corresponding increase in the diameter of the aorta and vena cava proximal to the fistula; the dimensions of the vessels distally remain unchanged.77
Clinical Presentation
Most AVFs encountered by vascular surgeons are of the chronic variety; exceptions are traumatic AVFs and spontaneous AVFs that develop as a result of ruptured aneurysms. It is estimated that 49% to 66% of trauma patients with AVFs present acutely and require immediate repair. In the remaining patients, the diagnosis is missed initially and the presentation is delayed days, weeks, or years after the initial injury.10,11,39,41
History
The most common presenting symptoms of an AVF are a thrill or bruit (61%-74% of cases) or a pulsatile mass (20%-32% of cases).11 Large fistulas may cause symptoms by diverting arterial blood away from the distal vascular bed. Consequently, patients may experience symptoms of arterial insufficiency that mimic those caused by arterial stenosis. Regional and local symptoms of venous hypertension may also be present in patients with large AVFs. An antecedent history of penetrating or iatrogenic trauma (renal or hepatic biopsy, central venous catheter placement, previous cardiac or vascular intervention), lumbar disk surgery, or preexisting AAA should be elicited. Symptoms of CHF, gastrointestinal bleeding, hematuria, and massive leg swelling, characteristic of large aortocaval, ilioiliac, arterioportal, or renal fistulae, should be ascertained.
Physical Examination
Local manifestations can include both venous and arterial signs. Venous findings range from limb swelling with dilated veins to varicose veins, pigmentation, and ulceration in more chronic cases. Ipsilateral limb ischemia of varying severity is rare but can occur with both central and peripheral AVFs. Whether symptoms of leg ischemia develop depends on the size of AVFs, as well as embolization to the runoff vessels.34,83 All peripheral pulses should be evaluated.
The only findings in patients with small fistulae may be a bruit or thrill beneath a scar or over a pulsatile mass. A careful search should be made for scars and incisions from previous trauma or operations. In patients with extremity AVFs, temporary compression of the artery proximal to the fistula elicits the Nicoladoni or Branham sign, consisting of slowing of the heart rate and a reduction in pulse pressure. An abnormal aortic pulsation and an epigastric bruit or thrill are suggestive of an intraabdominal AVF. Auscultation over the aorta, kidneys, liver, and spleen for the presence of a holosystolic murmur should be performed routinely.
The symptoms and signs of CHF—elevated jugular venous pressure, tachycardia, low diastolic blood pressure, pulmonary rales, cardiomegaly with an S3 cardiac gallop rhythm, hepatomegaly, and peripheral edema—are present in up to a third of individuals with acquired AVFs.68–70 Although most common in aortocaval and aortorenal AVFs, CHF may also be the presenting symptom in patients with high-flow iliac, femoral, or carotid AVFs.
Specific Locations
Neck and Upper Extremity
A rapidly expanding hematoma, carotid bruit or thrill, and dilated neck veins suggest the diagnosis of a carotid-jugular AVF. Ischemic neurologic symptoms directly attributable to the carotid AVF may result from embolization or shunting of blood through the fistula.9,22 The clinical diagnosis of vertebral AVFs is often unsuspected; only half of patients present with overt signs and symptoms of vertebral artery injury, and ischemic neurologic symptoms are rare. Subclavian, axillary, and brachial AVFs may manifest with diminished or absent peripheral pulses, abnormal forearm blood pressure measurements, arm swelling, dilated veins, hand ischemic symptoms, and a pulsatile mass with a thrill and bruit. Radial and ulnar AVFs are usually asymptomatic and manifest as a thrill or bruit over a small pulsatile mass.
Aortoiliac
Abdominal or back pain is the presenting symptom in 70% to 80% of patients with spontaneous AVFs involving the aorta and iliac arteries. A pulsatile mass or pronounced aortic pulsation is usually present. In 75% of cases, there is a holosystolic murmur, but a thrill may be palpable in only about 25% of patients. Symptoms of CHF, including fatigue, shortness of breath with exertion, anorexia, nausea, leg swelling, oliguria, hematuria, and rectal bleeding due to rupture of mucosal veins in the bladder and rectum, may be present in 11.5% to 35% of cases. Patients with traumatic AVFs are usually young, healthy males with a history of trauma weeks, months, or years previously.50,67–70
Renal and Splanchnic
Patients with spontaneous aortorenal fistulae may present with abdominal pain, hypertension, hematuria, varicocele, non–contrast-enhancing “silent” left kidney, CHF, and rarely, renal insufficiency.70,72 Small renal AVFs are usually discovered incidentally, with a bruit over the kidney the only evidence of their presence. Blood flow through larger renal AVFs is estimated to approach 60% to 82% of the total ipsilateral renal blood flow.45 With such high flow rates through the fistula, a significant portion of the kidney perfused by the involved artery may become ischemic, leading to hypertension.
The presentation of patients with symptomatic splanchnic AVFs includes abdominal pain, mesenteric ischemia, gastrointestinal hemorrhage, and symptoms and signs of portal hypertension. Patients who develop AVFs associated with lumbar disk surgery usually manifest symptoms within days or weeks after the procedure, and the clinical presentation may range from a lower abdominal holosystolic murmur with a small, occult fistula to massive leg swelling, signs of CHF, and an abdominal bruit with a large fistula.
Lower Extremity
An expanding hematoma, bruit or thrill, and newly developed lower extremity edema following trauma or interventional procedures suggest the presence of lower extremity AVFs. Most iatrogenic AVFs are small and will seal spontaneously. Certain risk factors, such as large communications, connections at branch points, and anticoagulation, may prevent closure of these procedure-related AVFs. Long-standing AVFs can lead to lower extremity arterial insufficiency due to a “steal” phenomenon. However, more frequent findings include low extremity venous insufficiency secondary to venous hypertension, high-output cardiac failure resulting from increased venous return, or aneurysmal degeneration of the artery. Table 86-1 summarizes the specific vascular injuries and associated incidence and presentation of common peripheral AVFs.
Table 86-1
Specific Vascular Injuries and Arteriovenous Fistulae (AVFs)
| Artery Injured | AVFs (% of arterial injuries) | Clinical Presentation |
| Carotid | 4-27 | Pulsatile mass, thrill, bruit, embolic or ischemic neurologic symptoms, dilated neck veins |
| Subclavian/axillary | 4.1-23 | Pulsatile mass, thrill, bruit, dilated arm veins, nerve and pulse deficits |
| Vertebral | 2-7 | Pulsatile mass, bruit, pulsatile tinnitus, neurologic symptoms (rare) |
| Brachial | 8.3-9 | Pulsatile mass, thrill, bruit, dilated veins, digital or arm swelling, pulse deficits |
| Iliac | 0.4-1.4 | Congestive heart failure, lower extremity edema, bruit, limb ischemia |
| Femoral* | 12-30 | Pulsatile mass, bruit, leg swelling, limb ischemia, congestive hear failure |
| Popliteal | 5-16 | Pulsatile mass, thrill, bruit, leg swelling, dilated veins, venous stasis ulceration, nerve and pulse deficits |
| Tibioperoneal | 2-35 | Pulsatile mass, thrill, bruit, leg swelling, nerve and pulse deficits |
* Common, superficial, and deep femoral arteries combined.
Diagnostic Imaging
Plain chest and abdominal radiographs should be obtained in all patients with suspected AVFs to look for evidence of CHF and prior trauma, such as shotgun pellets or bullet fragments. Rarely, calcification of a traumatic AVF may be present. Ankle-brachial indices are determined and venous and carotid duplex imaging is performed as indicated by the patient’s symptoms. Cardiac evaluation should include an electrocardiogram and echocardiography. Cardiac catheterization is not routinely necessary and is reserved for cases of diagnostic uncertainty. The typical cardiac catheterization findings in patients with well-established AVFs include an increased cardiac output and elevated right atrial, right ventricular, and pulmonary artery wedge pressures, with a corresponding decrease in peripheral vascular resistance. The oxygen saturation in the veins proximal to the fistula is increased and may approach that of the artery. Routine blood chemistries are obtained to evaluate renal and hepatic function, brain natriuretic peptide levels, a complete blood count, and urinalysis. Sufficient blood and blood products should also be made available if surgical repair is being considered.84
Color-Flow Duplex Imaging
Duplex findings characteristic of AVFs include visualization of the fistulous connection, a color mosaic at the level of the fistula, and the presence of color pixels in the soft tissues adjacent to the fistula. Loss of triphasic waveforms in the artery proximal to the fistula, decreased flow in the distal artery, and continuous high-velocity flow in the vein cephalad to the fistula are the usual pulse Doppler findings (Fig. 86-4).44,45,85 However, the use of color-flow duplex to evaluate vascular trauma and AVFs has limits. Although such imaging is useful in detecting AVFs and other arterial injuries, its sensitivity for detecting correctable defects such as intimal flaps is low compared with that of computed tomographic angiography (CTA) or angiography.84 The accuracy of the study is also highly dependent on technician skill, and in the acute setting, open and bleeding wounds, subcutaneous air, hematoma, and fractures limit its application. Patient body mass index and interference from anatomic structures also make visualization difficult in certain locations. No data are available to address the sensitivity and specificity of duplex imaging for the diagnosis of acquired AVF.
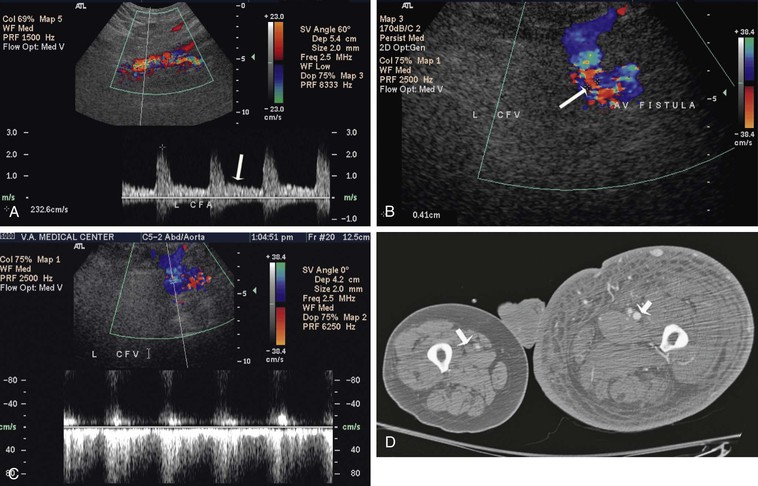
Figure 86-4 Ultrasound image and CT scan of a patient with a large common femoral AVF. Loss of triphasic waveform is observed in the common femoral artery (CFA) proximal to the fistula (A); a large connection is demonstrated by the white arrow between the CFA and common femoral vein (CFV) (B); a pulsatile and reverse flow is seen in the CFV distal to the fistula (C); and early enhancement of superficial femoral vein (white arrow) on the side with an AVF (D).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


