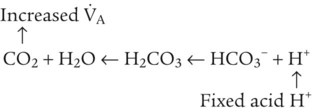
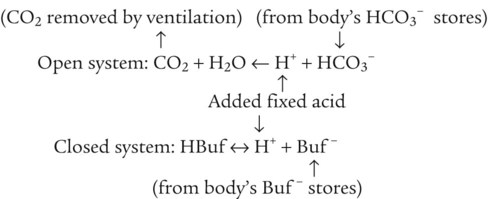
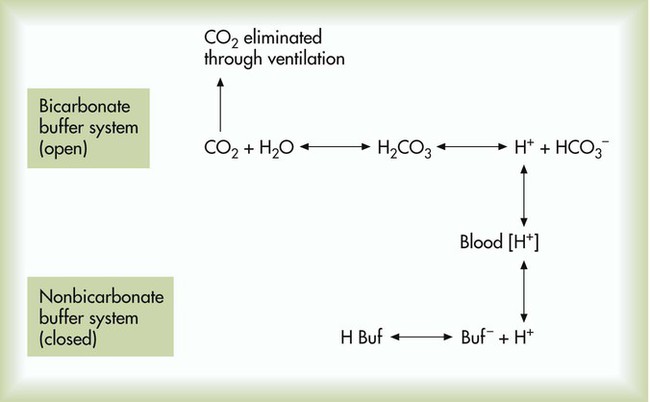
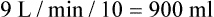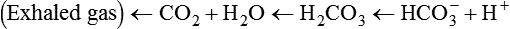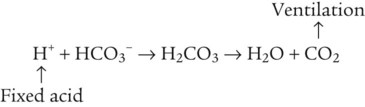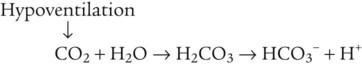After reading this chapter you will be able to: As CO2 diffuses into the blood at the tissue level, this reaction occurs primarily in the erythrocyte where it is catalyzed by carbonic anhydrase, an intracellular enzyme. In a process called isohydric buffering,1 most H+ produced in this fashion causes no change in pH because hemoglobin (Hb) in the erythrocyte immediately buffers the H+. When blood reaches the lungs, Hb releases H+ to form CO2 as shown: Catabolism of proteins continually produces fixed (nonvolatile) acids such as sulfuric and phosphoric acids. In addition, anaerobic metabolism produces lactic acid. In contrast to carbonic acid, these nonvolatile acids are not in equilibrium with a gaseous component. However, H+ of fixed acids can be buffered by bicarbonate ions (HCO3−) and converted to CO2 and water (H2O) (see the previous reaction); the CO2 formed is eliminated in exhaled gas. Compared with daily CO2 production, fixed acid production is small, averaging only about 50 to 70 mEq/day.2 Certain diseases, such as untreated diabetes, increase fixed acid production. Hydrogen ions produced in this way stimulate respiratory centers in the brain. The resulting increase in ventilation eliminates more CO2, pulling the hydration reaction to the left: Box 13-1 summarizes the characteristics and components of bicarbonate and nonbicarbonate buffer systems. Open and closed buffer systems play different roles in buffering fixed and volatile acids, and they differ in their ability to function in wide-ranging pH environments. Volatile acid (H2CO3) accumulates only if ventilation cannot eliminate CO2 fast enough to keep up with the body’s CO2 production. In such a case, the reaction between CO2 and H2O moves continually to the right, creating more H2CO3 and, ultimately, more H+ and HCO3−. The HCO3− produced in this way is incapable of buffering the H+ with which it was coproduced. The only buffer system that can buffer the H+ of volatile acid is the nonbicarbonate buffer system. Both nonbicarbonate and bicarbonate buffer systems can buffer the H+ produced by fixed acids; this is true of the bicarbonate buffer system only if ventilation is not impaired and CO2 can be adequately eliminated. Both systems are physiologically important, each playing a unique and essential role in maintaining pH homeostasis. Table 13-1 summarizes the approximate contributions of various blood buffers to the total buffer base. Bicarbonate buffers have the greatest buffering capacity because they function in an open system. TABLE 13-1 Individual Buffer Contributions to Whole Blood Buffering From Beachey W: Respiratory care anatomy and physiology: foundations for clinical practice, ed 2, St Louis, 2007, Mosby. Bicarbonate and nonbicarbonate buffer systems do not function in isolation from one another but are intermingled in the same solution (whole blood), in equilibrium with the same [H+] (Figure 13-1). Increased ventilation increases the CO2 removal rate, causing nonbicarbonate buffers (Hbuf) to release H+. Decreased ventilation ultimately causes Hbuf to accept more H+. [H+] can be calculated by algebraic rearrangement of this equation, as follows: pH is a logarithmic expression of [H+], and the term 6.1 is the logarithmic expression of the H2CO3 equilibrium constant. Because dissolved carbon dioxide (Pco2 × 0.03) is in equilibrium with and directly proportional to blood [H2CO3], and because blood Pco2 is more easily measured than [H2CO3], dissolved CO2 is used in the denominator of the H-H equation. The H-H equation is specific for calculating the pH of the bicarbonate buffer system of the blood. The calculation of this pH is important because it equals the pH of blood plasma; because all buffer systems in the blood are in equilibrium with the same pH, the pH of one buffer system is the same as the pH of the entire plasma solution (the isohydric principle).1 The functions of bicarbonate and nonbicarbonate buffer systems are summarized in Table 13-2. TABLE 13-2 From Beachey W: Respiratory care anatomy and physiology: foundations for clinical practice, ed 2, St Louis, 2007, Mosby. Table 13-1 lists the nonbicarbonate buffers in the blood. Of these, Hb is the most important because it is the most abundant. As mentioned, these buffers are the only ones available to buffer carbonic acid. However, they can buffer H+ produced by any acid, fixed or volatile. Because nonbicarbonate buffers (Buf−/HBuf) function in closed systems, the products of their buffering activity eventually accumulate, slowing or stopping further buffering activity:
Acid-Base Balance
 Describe how the lungs and kidneys regulate volatile and fixed acids.
Describe how the lungs and kidneys regulate volatile and fixed acids.
 Describe how the equilibrium constant of an acid is related to its ionization and strength.
Describe how the equilibrium constant of an acid is related to its ionization and strength.
 State what constitutes open and closed buffer systems.
State what constitutes open and closed buffer systems.
 Explain why open and closed buffer systems differ in their ability to buffer fixed and volatile acids.
Explain why open and closed buffer systems differ in their ability to buffer fixed and volatile acids.
 Explain how to use the Henderson-Hasselbalch equation in hypothetical clinical situations.
Explain how to use the Henderson-Hasselbalch equation in hypothetical clinical situations.
 Describe how the kidneys and lungs compensate for each other when the function of one is abnormal.
Describe how the kidneys and lungs compensate for each other when the function of one is abnormal.
 Explain how renal absorption and excretion of electrolytes affect acid-base balance.
Explain how renal absorption and excretion of electrolytes affect acid-base balance.
 Classify and interpret arterial blood acid-base results.
Classify and interpret arterial blood acid-base results.
 Explain how to use arterial acid-base information to decide on a clinical course of action.
Explain how to use arterial acid-base information to decide on a clinical course of action.
 Explain why acute changes in the carbon dioxide level of the blood affect the blood’s bicarbonate ion concentration.
Explain why acute changes in the carbon dioxide level of the blood affect the blood’s bicarbonate ion concentration.
 Calculate the anion gap and use it to determine the cause of metabolic acidosis.
Calculate the anion gap and use it to determine the cause of metabolic acidosis.
 Describe how standard bicarbonate and base excess measurements are used to identify the nonrespiratory component of acid-base imbalances.
Describe how standard bicarbonate and base excess measurements are used to identify the nonrespiratory component of acid-base imbalances.
 State how Stewart’s strong ion difference approach to acid-base regulation differs from the Henderson-Hasselbalch approach.
State how Stewart’s strong ion difference approach to acid-base regulation differs from the Henderson-Hasselbalch approach.
Hydrogen Ion Regulation in Body Fluids
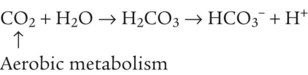
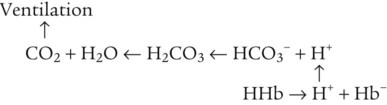
Bicarbonate and Nonbicarbonate Buffer Systems
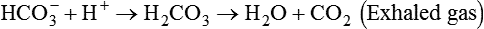
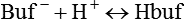
Buffer Type
Total Buffering (%)
Bicarbonate
Plasma bicarbonate
35
Erythrocyte bicarbonate
18
Total bicarbonate buffering
53
Nonbicarbonate
Hemoglobin
35
Organic phosphates
3
Inorganic phosphates
2
Plasma proteins
7
Total nonbicarbonate buffering
47
Total
100
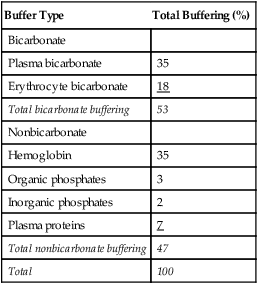
pH of a Buffer System: Henderson-Hasselbalch Equation
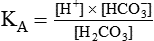
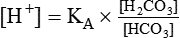
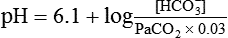
Physiologic Roles of Bicarbonate and Nonbicarbonate Buffer Systems
Buffer
Type of System
Acids Buffered
Bicarbonate
Open
Fixed (nonvolatile)
Nonbicarbonate
Closed
Volatile (carbonic)
Fixed
Nonbicarbonate Buffer System
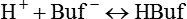
Acid-Base Balance

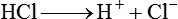
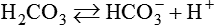
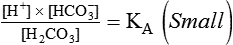
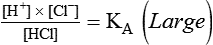
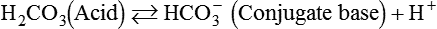
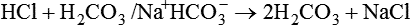
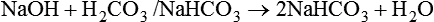
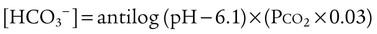
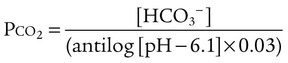
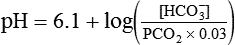
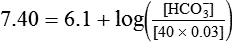
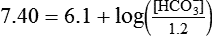
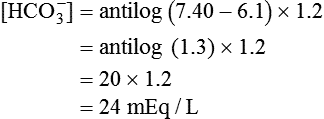
 ) of 8 L/min. The patient’s PaCO2 is 55 mm Hg, pH is 7.30, and bicarbonate is 26 mEq/L, and the therapist wishes to maintain a pH of 7.35. How much does the RT need to change the PaCO2 to achieve this desired pH, and what change in the patient’s VT does this require?
) of 8 L/min. The patient’s PaCO2 is 55 mm Hg, pH is 7.30, and bicarbonate is 26 mEq/L, and the therapist wishes to maintain a pH of 7.35. How much does the RT need to change the PaCO2 to achieve this desired pH, and what change in the patient’s VT does this require?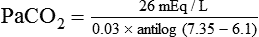
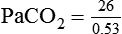
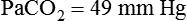
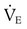 required to produce a PaCO2 of 49 mm Hg. Because
required to produce a PaCO2 of 49 mm Hg. Because 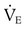 is inversely proportional to PaCO2, the following can be stated:
is inversely proportional to PaCO2, the following can be stated: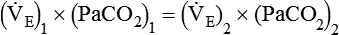
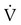 )2 as follows:
)2 as follows: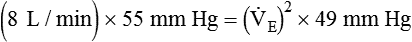
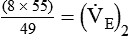
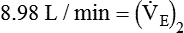
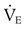 from 8 L/min to approximately 9 L/min yields a PaCO2 of 49 mm Hg and a pH of approximately 7.35. Now the RT divides the new
from 8 L/min to approximately 9 L/min yields a PaCO2 of 49 mm Hg and a pH of approximately 7.35. Now the RT divides the new 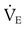 of 9 L/min by the respiratory frequency to calculate the new VT required:
of 9 L/min by the respiratory frequency to calculate the new VT required: