Access for Aortic Valve Surgery: Minimally Invasive Open Approaches
Danielle A. Smith
S. Chris Malaisrie
Introduction
The conventional approach to aortic valve replacement (AVR) is through a full sternotomy. Any approach that does not involve a full sternotomy is considered a minimally invasive approach. The first minimally-invasive aortic valve replacement (MIAVR) was described in 1993 and performed through a right thoracotomy. By 1996, the spectrum of minimally invasive techniques also included hemisternotomies, transverse sternotomies, as well as a parasternal incision. Currently, anterior right thoracotomy (ART) and upper hemisternotomy (UHS) are the predominant MIAVR approaches.
A direct correlation between clinical outcomes and institutional surgical volume is well established for conventional AVR. Accordingly, incorporation of MIAVR into surgical practice requires established expertise in conventional aortic valve surgery and adequate case volume. A caseload of 30 or more aortic valve-related cases per year is suggested. Moreover, open communication between the cardiac anesthesiologist, the surgeon, perfusionist, and operating room nurses is essential and the lack of a dedicated team is a contraindication to MIAVR. The requirements include an experienced cardiac anesthesiologist adept with transesophageal echocardiography (TEE). Experience with placement of transjugular coronary sinus catheters is an added benefit. The perfusionist should be familiar with noncentral cannulation techniques and utilization of vacuum- or kinetic-assisted venous drainage during cardiopulmonary bypass (CPB) when small cannulae are selected. Finally, the operating room nurses should be familiar with low-profile retractors, and long-shafted instruments that greatly facilitate MIAVR.
Patient selection is essential to a successful operation. Longer CPB and aortic cross-clamp (ACC) times can be expected during MIAVR, without a negative effect on postoperative outcomes. Indications for MIAVR are similar to those for isolated AVR through a full sternotomy and include aortic stenosis, aortic regurgitation, and benign aortic valve tumors. Any patient requiring an isolated AVR is, therefore, a candidate for MIAVR. The only absolute contraindication to MIAVR is the inability to tolerate increased ACC and CPB times. For example, some patients with severe heart failure or chronic lung disease may not tolerate even minimally increased operating times.
Several relative contraindications to MIAVR should be considered. Morbid obesity is a relative contraindication to MIAVR if adequate exposure is not feasible. However, with the appropriate minimal access retractors, sufficient exposure can be achieved. Patients with chest wall deformities, such as pectus excavatum and kyphoscoliosis, should be approached with caution when considering MIAVR. In some patients, UHS should be avoided if the deformity alters the normal position of the heart, deviating the surgical field into either pleural cavity. Previous right chest surgery is a relative contraindication for ART given the likelihood of dense pleural adhesions.
UHS is the first logical approach for surgeons starting an MIAVR practice as it can be easily converted to a full sternotomy. Once UHS is mastered, ART may be considered. Surgeons should have experience with peripheral CPB prior to incorporation of the ART approach. UHS is the recommended approach for reoperative AVR in order to safely cross-clamp the aorta and avoid injury to adherent structures such as the pulmonary artery (PA), left atrial appendage, and any pre-existing bypass grafts. The ART approach is best suited for patients who may have difficulty following sternal precautions or who otherwise wish to avoid sternotomy. A thoracotomy is cosmetically best through an incision slightly superior to the inframammary crease (and not directly through the crease). The entire incision can then be moved to the desired interspace, which is typically more cephalad. An incision directly into the third or fourth interspace, that is well above the inframammary fold, may be disfiguring, particularly for a female patient.
Patients who require concomitant cardiac surgery such as coronary artery bypass grafting (CABG), mitral/tricuspid valve surgery, ascending aortic replacement, or atrial fibrillation (AF) ablation procedures are not ideal candidates for MIAVR. In principle, the cardiac operation itself should not be compromised in order to pursue a minimally invasive approach, and a complex, but complete, operation may be better suited through a full sternotomy. Nevertheless, limited CABG to the right coronary artery, double valve surgery, AVR with ascending aortic replacement, and AVR with AF ablation procedures have been successfully performed with a minimally invasive approach. Moreover, a hybrid approach may be considered, in particular for patients requiring coronary revascularization. Coronary artery stenting may be performed before or after MIAVR, depending on clinical presentation.
Preoperative planning should include imaging to determine the appropriate location for the UHS or ART incision and to evaluate the peripheral vasculature if peripheral cannulation is planned. Peripheral arterial cannulation may be beneficial in ART where direct aortic cannulation is remote from the incision. It is important for the surgeon to have a plan for myocardial protection, which may include adjunctive techniques in percutaneous cardioplegia.
Preoperative Work-Up
Preoperative evaluation for MIAVR requires careful attention to several pre-existing conditions: Peripheral vascular disease (PVD), chronic obstructive pulmonary disease (COPD), a history of chest wall irradiation or deformity, and previous cardiac surgery.
In patients with PVD, the risk of stroke or systemic embolization is significantly elevated with retrograde arterial perfusion. Computed tomography (CT) angiography of the chest, abdomen, and pelvis should be done routinely if retrograde arterial perfusion is planned. It is important to assess severity, location, and nature of atherosclerosis. Plaque that is smooth and calcified poses less of a danger than soft or irregular lesions. Consideration must also be taken of the size and tortuosity of the iliofemoral vessels when selecting the appropriate arterial cannula. Moreover, patients who undergo preoperative coronary catheterization are at risk for iatrogenic dissection of the iliac artery during femoral access. Occult dissection is usually confined to a tortuous segment as it emerges from the pelvis. However, if unrecognized, acute dissection into the aorta can be induced by retrograde arterial perfusion. To avoid this complication, CT angiography should be performed after cardiac catheterization when femoral artery cannulation is anticipated.
The chest deformity seen in severe COPD may alter the anatomic relationship between the chest wall and aortic valve. CT allows visualization of the anatomic structures within the chest and provides crucial information about their spatial relationships. CT is also useful for evaluation of the pleural space and the extent of lung disease. It is important to determine the distance between the posterior sternal table and the right ventricle, preoperatively, in patients with previous cardiac surgery or chest wall irradiation. This can be achieved with noncontrast chest CT. In patients with previous CABG, patent internal mammary artery grafts crossing the midline are particularly hazardous. When planning ART, a careful history must be taken of recurrent pneumonia, pneumothorax, and right lung resection, as these may be associated with dense pleural adhesions.
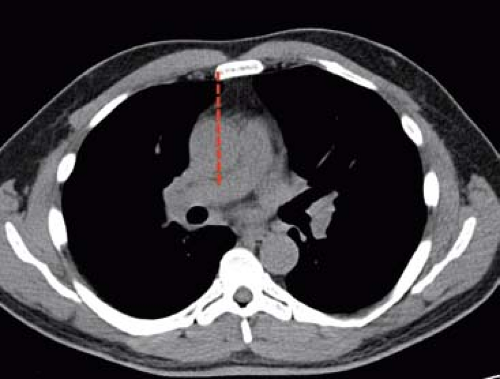
Finally, the added benefit of preoperative CT imaging also includes preoperative planning of the incision for UHS with direct ascending aortic cannulation, to evaluate the severity and distribution of aortic calcification. This information is helpful in the formulation of cannulation and cross-clamp strategy. In bicuspid aortic valve disease, CT may be used to measure the aortic root and ascending aorta to determine the presence of aortic aneurysm that will alter the approach. Chest CT is also helpful in determining which patients are best candidates for ART. A vertical line is drawn from the right sternal border through the ascending aorta in the axial CT view (Fig. 6.1). If more than one-half of the ascending aorta is to the right of this line, ART is appropriate. From the CT, the preferred intercostal space for both ART and UHS can be determined. The ideal interspace is marked by the tip of the right atrial appendage.
Preparation for Cannulation and Cardioplegia
The surgeon must devise a plan for cannulation and administration of cardioplegia. Peripheral cannulation should be considered for reoperative MIAVR given the risk of
adhesions and the need to quickly place the patient on bypass if uncontrolled hemorrhage occurs. Cannulation options include the right axillary artery and the common femoral artery. At the very least, a wire should be placed in the common femoral artery, prior to sternotomy. UHS should provide ample exposure for placement of an arterial cannula in the ascending aorta. Although venous cannulation through the UHS incision is easily accomplished, percutaneous femoral venous cannulation is preferred to maximize exposure through the small incision. Aortic cannulation via ART, however, can be challenging and the procedure is more easily performed using open cannulation of the common femoral artery and vein.
adhesions and the need to quickly place the patient on bypass if uncontrolled hemorrhage occurs. Cannulation options include the right axillary artery and the common femoral artery. At the very least, a wire should be placed in the common femoral artery, prior to sternotomy. UHS should provide ample exposure for placement of an arterial cannula in the ascending aorta. Although venous cannulation through the UHS incision is easily accomplished, percutaneous femoral venous cannulation is preferred to maximize exposure through the small incision. Aortic cannulation via ART, however, can be challenging and the procedure is more easily performed using open cannulation of the common femoral artery and vein.
Cardioplegia may be given using antegrade or retrograde delivery. Antegrade cardioplegia is given initially through the aortic root and can be subsequently delivered directly into the right and left main coronary ostia after aortotomy. Alternatively, retrograde cardioplegia may be given after the initial antegrade dose. For both UHS and ART, the retrograde catheter can be placed directly through the right atrium and directed into the coronary sinus under TEE guidance. To maximize exposure and minimize cannula clutter, a percutaneous transjugular coronary sinus catheter may be placed under TEE and fluoroscopic guidance by an experienced cardiac anesthesiologist, prior to incision. The decision to use a transjugular coronary sinus catheter must be appropriately communicated to the anesthesiologist prior to the operation. With an experienced team, catheter placement should not add more than 15 minutes to the total operating room time.
Positioning
For both UHS and ART, the patient is placed in the supine position with arms tucked at the sides. External defibrillator pads are placed on the patient. The surgeon should confirm with the anesthesia team that the pulse oximeter and peripheral arterial and venous lines are functional. The incision should be marked on the skin. The patient is then prepped and draped to include the chest incision and the groins.
Intraoperative Monitoring, Lines, and Ventilation
Intraoperative TEE is used routinely in MIAVR. A PA catheter may be indicated depending on the patient’s risk factors. If peripheral arterial cannulation is planned, limb perfusion can be monitored using pulse oximetry of the lower extremities for femoral cannulation or with an ipsilateral radial arterial line during axillary cannulation. To reduce the number of cannulae in the operative field, the right atrial venous cannula should be placed percutaneously through the common femoral vein. In addition, both the retrograde cardioplegia catheter and PA vent may be placed percutaneously through the jugular vein. An experienced cardiac anesthesiologist usually performs this procedure, which requires TEE imaging supplemented with portable fluoroscopy.
A single-lumen endotracheal tube is standard for both ART and UHS exposures. The right lung may be retracted posteriorly in ART to achieve optimal visualization without single-lung ventilation. Due to the smaller caliber of peripheral cannulae, vacuum- or kinetic-assisted venous drainage is used to facilitate emptying of the heart for peripheral CPB.
Technique
Upper Hemisternotomy Approach
For surgeons who are not yet experienced in MIAVR, the J-shaped UHS is the recommended initial approach. UHS has the advantage of not requiring any specialized equipment. It can be applied to both primary and reoperative surgery, with either central or peripheral CPB. Precise incision placement is assisted by preoperative chest CT, which
identifies the aortic valve relative to the surface anatomy of the bony thorax. A 5 to 8 cm vertical skin incision is made just caudal to the sternal angle of Louis (Fig. 6.2A). Subcutaneous tissue is dissected up to the sternal notch superiorly and down to the intercostal space inferiorly, without requiring extension of the skin incision. The J-shaped hemisternotomy is mapped out along the center of the sternum into the right third or fourth interspace (Fig. 6.2B), depending on preoperative imaging, and is scored using electrocautery. For patients without a preoperative chest CT, and for surgeons new to MIAVR, the fourth interspace should be used to optimize exposure.
identifies the aortic valve relative to the surface anatomy of the bony thorax. A 5 to 8 cm vertical skin incision is made just caudal to the sternal angle of Louis (Fig. 6.2A). Subcutaneous tissue is dissected up to the sternal notch superiorly and down to the intercostal space inferiorly, without requiring extension of the skin incision. The J-shaped hemisternotomy is mapped out along the center of the sternum into the right third or fourth interspace (Fig. 6.2B), depending on preoperative imaging, and is scored using electrocautery. For patients without a preoperative chest CT, and for surgeons new to MIAVR, the fourth interspace should be used to optimize exposure.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree