Abnormal Systemic Venous Connections
Tal Geva
The word vein stems from the Latin vena (from the Latin verb venio, “to come”). Hence, a vein is a passage, a conduit, a vessel that brings blood to the heart regardless of the consistency of the blood it carries. A single exception is the portal vein, which carries blood from the intestine to the liver before its return to the heart through the hepatic veins. The spectrum of systemic venous anomalies varies widely from minor asymptomatic anatomic variations to complex abnormalities that can lead to cyanosis or that might complicate surgical repair of congenital heart disease. Clinically significant abnormalities of the systemic veins are infrequent when visceroatrial situs is lateralized (either situs solitus or situs inversus). In contrast, the incidence of systemic venous anomalies in patients with heterotaxy syndrome exceeds 90% (1,2). The advent of pediatric cardiac surgery and interventional cardiology heightened interest in these anomalies. Consequently, in-depth understanding and accurate diagnosis of abnormal systemic venous connections have become essential for successful management of many congenital cardiovascular anomalies.
As with all congenital heart disease, understanding the anatomy of the systemic veins and its variations is greatly facilitated by reviewing its embryology. In considering the wide range of abnormalities of the systemic veins, an anatomic classification based on developmental principles provides a solid framework. In this chapter, the embryology of the systemic veins is reviewed first to provide a background for further discussion of specific venous anomalies. These have been categorized into the following groups: anomalies of the superior venae cavae, anomalies of the coronary sinus, anomalies of the inferior vena cava (IVC), anomalies of the ductus venosus, and anomalies of the sinoatrial valves.
Embryology
There are three basic venous systems in the human embryo: (a) the cardinal veins and their tributaries, which form the superior and inferior caval systems; (b) the umbilical, vitelline, and omphalomesenteric veins, which carry the blood from the placenta, yolk sac, and intestine; and (c) the pulmonary veins, which return the blood from the lungs.
The age of human embryos cannot be estimated reliably on the basis of their length, which may vary greatly (3), or on the number of somites, which are visible for only a limited time. In 1942, therefore, Streeter (4) proposed classifying human embryos into 25 age groups, or horizons, each representing 2 days of embryonic life. He later omitted the last two stages because he thought that stage XXIII could be considered the last stage of embryonic development. This approach generally has been accepted.
Normal Development of the Cardinal and Umbilicovitelline Venous Systems
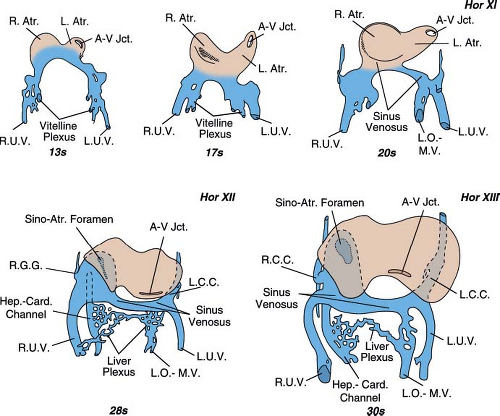
The sinus venosus, that is, the cavity into which all veins eventually drain, develops by enlargement of the confluence of the umbilical veins and joins the atrial segment of the heart through a slit-like opening, the sinoatrial foramen. It already is present in the 20 somite embryo (horizon XI) (Fig. 36.1). It is composed of a middle section, which has been called the transverse sinus, and right and left sections, which have been named the right and left horns of the sinus venosus. The three main paired venous systems of the embryo—the cardinal, the umbilical, and the vitelline veins—drain into the ipsilateral horns of the sinus venosus.
The right and left umbilical veins develop first. They can be recognized during the third week of gestation in embryos of 13 somites (Fig. 36.1). At the same time, the vitelline plexus of the liver is formed and soon becomes more prominent on the right side. It will connect with the sinus venosus through the right hepatocardiac channel, which drains into the right horn of the sinus venosus and with the yolk sac via the left omphalomesenteric vein (Fig. 36.1).
The anterior cardinal veins make their appearance shortly after the umbilical and the vitelline veins (horizon XI, 22 to 24 days of gestation) (Fig. 36.2 4 weeks). They drain blood from the fused neural folds that form the central nervous system. Soon after, the posterior cardinal veins appear lateral to the spinal cord. They join the anterior cardinals to form the right and left common cardinals (ducts of Cuvier) and drain together with the umbilical and vitelline veins into the right and left horns of the sinus venosus. Each horn of the sinus venosus drains for a short time into its respective sides of the common atrium (Fig. 36.1, stage of 20 somites).
At the beginning of the fourth week of embryonic life, an invagination appears between the left horn of the sinus venosus and the left atrium (LA) and ultimately separates the left horn from the LA. A rightward shift of the transverse portion of the sinus venosus that follows the appearance of the aforementioned invagination will complete the anatomic isolation of the LA from the three pairs of veins that enter the sinus venosus (cardinals, umbilicals, and vitellines). These veins will now drain into the right atrium (RA) through the sinoatrial foramen (Fig. 36.1, stage of 28 somites).
Normal Development of the Right Superior Vena Cava and the Coronary Sinus
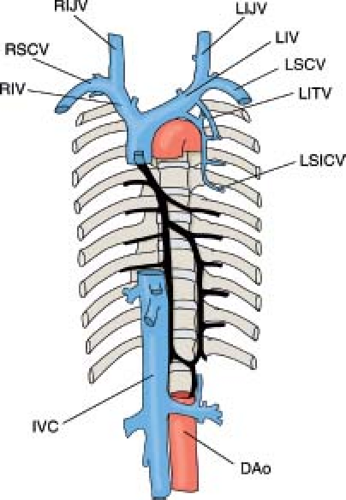
The right superior vena cava (RSVC) extends from the confluence of the right and left innominate veins (RIV, LIV) to the RA. It is composed of the most proximal part of the right anterior cardinal vein and the right common cardinal vein. The development of the LIV at the seventh week of gestation usually is followed by the involution of the left SVC (LSVC), which becomes the ligament of Marshall (5). As the transverse segment of the sinus venosus shifts rightward, it pulls the left horn of the sinus venosus along the posterior atrioventricular groove. The left horn of the sinus venosus and the adjacent part of the common cardinal vein receive the cardiac veins and form the coronary sinus. The mode of formation of the coronary sinus is responsible for the following anatomic
observations, which are helpful in making a diagnosis of certain congenital heart defects:
observations, which are helpful in making a diagnosis of certain congenital heart defects:
The orifice of a normally formed coronary sinus is always in the anatomically right atrium.
A persistent LSVC always continues with the coronary sinus since the left common cardinal vein is part of the coronary sinus and of the LSVC.
Functional connection (i.e., drainage) of a persistent LSVC or any other vein with the anatomically LA can occur only if the coronary sinus is partly or completely unroofed.
Normal Development of the Inferior Vena Cava
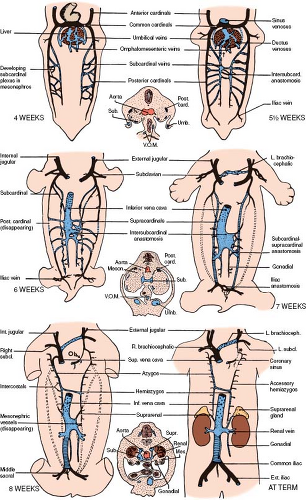
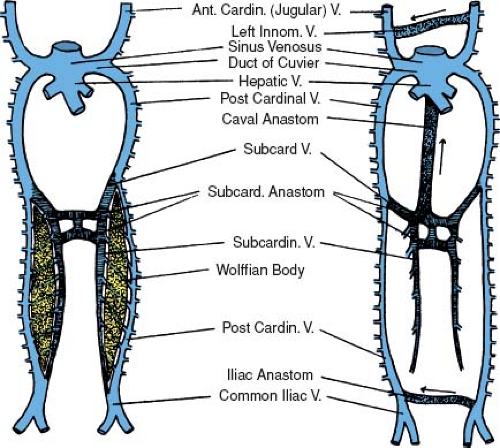
The IVC, the largest vein of the human body, is formed by the contribution of five different venous systems. The following description is a simplified version of a rather complex process. The posterior cardinal veins appear first, shortly after the establishment of the anterior cardinal veins. They develop as longitudinal vessels running in the dorsolateral portion of the urogenital fold and are associated primarily with the mesonephroi (wolffian bodies) (Figs. 36.2 and 36.3) (6). The subcardinal veins are a new venous plexus, which initiates the diversion of the venous
drainage of the mesonephroi and the developing urogenital system of the embryo. They are established along the ventromedial borders of the mesonephroi medial and ventral to the posterior cardinals (Fig. 36.3) (6). They have many channels connecting them with the posterior cardinals. As the mesonephroi increase in size and bulge toward the midline, the subcardinals are brought closer together. In the mid-mesonephric region, they establish communications with each other by several small vessels, which become confluent to form intersubcardinal anastomoses and eventually a large medial venous space, the subcardinal sinus (Figs. 36.2 5-1/2 weeks and 36.3). When the subcardinal sinus is established, the small vessels that connect the subcardinals with the posterior cardinals drain medially into the subcardinal sinus rather than laterally toward the posterior cardinals. This will result in the diminution and the later disappearance of the posterior cardinals at the level of the subcardinal sinus.
drainage of the mesonephroi and the developing urogenital system of the embryo. They are established along the ventromedial borders of the mesonephroi medial and ventral to the posterior cardinals (Fig. 36.3) (6). They have many channels connecting them with the posterior cardinals. As the mesonephroi increase in size and bulge toward the midline, the subcardinals are brought closer together. In the mid-mesonephric region, they establish communications with each other by several small vessels, which become confluent to form intersubcardinal anastomoses and eventually a large medial venous space, the subcardinal sinus (Figs. 36.2 5-1/2 weeks and 36.3). When the subcardinal sinus is established, the small vessels that connect the subcardinals with the posterior cardinals drain medially into the subcardinal sinus rather than laterally toward the posterior cardinals. This will result in the diminution and the later disappearance of the posterior cardinals at the level of the subcardinal sinus.
The blood from the posterior part of the body is still collected by the distal part of the postcardinals but returns to the heart by way of the subcardinal sinus (Figs. 36.2 5-1/2 weeks and 36.3). As the embryo grows, the blood volume that enters the subcardinal sinus increases and stimulates development of a new unpaired venous channel. This large unpaired venous channel, called the hepatic segment of the IVC, lies in a notch along the dorsal aspect of the liver (Fig. 36.2 6 weeks). It is formed by a confluence of the small vessels that appear in a fold of the dorsal body wall just to the right of the dorsal mesentery. This fold is known as the caval fold of the mesentery. Connections of these small vessels with the plexus of the liver cephalically and the mesonephroi via the subcardinal sinus caudally allows the blood to flow from the mesonephroi to the liver plexus, resulting in rapid enlargement of this channel (6). By the seventh week of gestation, this new unpaired venous channel connects the subcardinal sinus with the confluence of the hepatic veins and the ductus venosus. The resulting large vessel constitutes the suprahepatic segment of the IVC, which enters the RA (Fig. 36.4).
At this stage, the posterior cardinals have involuted to the level of the common cardinals proximally and the iliac veins distally. Dorsal to the subcardinal sinus, a new set of paired veins appear: the supracardinal veins (Fig. 36.2 6 weeks). These veins connect the subcardinal sinus with the cephalic remnant of the posterior cardinals via the azygos and hemiazygos veins. They also connect the subcardinal sinus with the iliac veins via the caudal remnant of the posterior cardinal veins. Eventually, the infrarenal portion of the left supracardinal vein will involute, and the infrarenal portion of the right supracardinal vein will become the infrarenal portion of the IVC (Fig. 36.2 7 weeks).
By the seventh week of gestation, venous channels have been established to connect the left side with the right side of the paired veins. Proceeding from cephalad to caudad, these channels are the LIV, the hemiazygos, the left renal vein, and the left common iliac (Fig. 36.2 7 weeks). The development of the connecting channels is followed by partial involution of the left side of the paired veins. All venous blood, with the exception of the pulmonary venous return, now enters the right-sided SVC and IVC, which bring it to the RA.
In summary, the five venous systems that contribute to the formation of the IVC, from distal to proximal, are the posterior cardinals, the right supracardinals, the subcardinals, the hepatic segment of the IVC, and the hepatic (former vitelline) veins. Four of these five venous systems begin as bilateral venous channels, a fact that
makes possible the existence of partly bilateral IVCs above the liver or below the kidneys (Fig. 36.5).
makes possible the existence of partly bilateral IVCs above the liver or below the kidneys (Fig. 36.5).
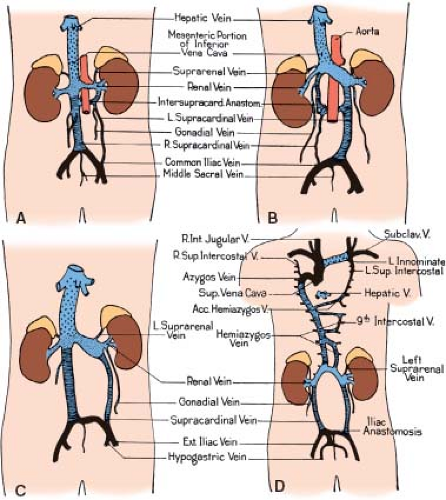 Figure 36.5 Variations in the formation of the IVC without abnormal systemic venous drainage. Drawings have been schematized as in Figure 36.2. A: Renal collar formed by persistent intersupracardinal anastomosis. B: Renal collar formed by a lumbar channel from left supracardinal. C: Double cava at lumbar level owing to persistent supracardinal veins on both sides. D: Absence of the hepatic segment of the IVC with azygos continuation into the right SVC. Acc., accessory; Ext., external; Int, internal; Subclav., subclavian; Sup., superior; V, vein. (From Patten BM. Human Embryology. 2nd ed. New York, NY: McGraw–Hill; 1953:637–681, with permission.) |
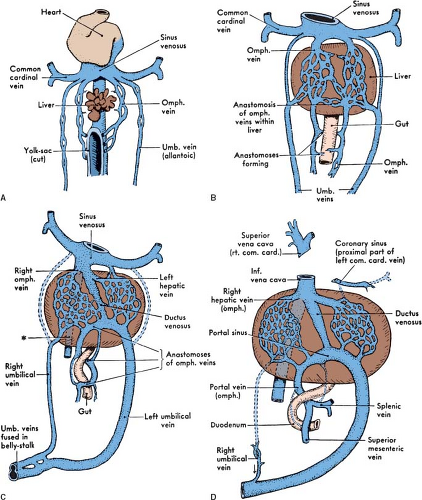
Normal Development of the Ductus Venosus
The paired umbilical veins, when first established, connect the placenta with the right and left horns of the sinus venosus. As the liver grows, it fuses with the lateral body wall. At the site of this fusion, multiple vessels develop, connecting the umbilical veins with the liver plexus (6). The umbilical stream tends to pass by way of these vessels into the liver, and the early direct connections of the umbilical veins with the sinus venosus involute. Distal to their entrance into the body of the embryo, the umbilical veins fuse. Consequently, there is only one vein in the umbilical cord. Inside the embryo, the right umbilical vein involutes except for a small segment that drains the body wall (Fig. 36.4). As the embryo grows and the volume of the umbilical venous blood increases, a new large channel is created through the liver substance, the ductus venosus, which connects the left umbilical vein with the right hepatic veins (Fig. 36.4). By the seventh week of gestation, placental blood finds its way to the RA by way of the left umbilical vein, the ductus venosus, and the suprahepatic segment of the IVC (Fig. 36.4). As it passes through the liver, the ductus venosus receives blood flow from the right and left hepatic veins and delivers it into the RA. After birth, the ductus venosus becomes the ligamentum venosum and the left umbilical vein becomes the ligamentum teres (round ligament of liver).
Azygos and Hemiazygos Veins
The azygos (“unpaired,” Greek) vein connects the suprarenal segment of the IVC with the right SVC (RSVC). It is formed by the suprarenal segment of the right supracardinal vein and the cephalic remnant of the right posterior cardinal vein (Fig. 36.2D–F). In the older litera ture, it was referred to as the vena azygos major. It commences from the right lumbar or the right renal vein or the IVC and passes through the aortic opening of the diaphragm to enter the thorax. It runs up medial to the thoracic vertebrae, to the right of the aorta and the thoracic duct, and receives the lower 10 right intercostal veins. At the level of the fourth thoracic vertebra, it arches anteriorly to connect with the posterior surface of the SVC (Fig. 36.6) (7).
The hemiazygos (“half azygos”) vein has two parts: The first is the left lower, or smaller, azygos vein (vena azygos minor), which starts at the lumbar region from one of the lumbar veins or from the left renal vein, passes through the left crus of the diaphragm, and ascends on the left side of the spine up to the level of the ninth thoracic vertebra (Fig. 36.6). It then turns to the right behind the aorta and the thoracic duct to terminate into the azygos vein. The left upper hemiazygos varies inversely with the size of the left superior intercostal vein. It receives the left intercostal veins that did not drain into the left superior intercostal vein and the lower hemiazygos and terminates in the right azygos or the lower hemiazygos. In some cases with bilateral SVCs, bilateral azygos veins may be present. It is obvious that the term bilateral azygos (i.e., bilaterally unpaired veins) is literally incorrect. It is also incorrect to use the term hemiazygos (i.e., half azygos) for a vein that extends from the suprarenal segment of the IVC to the LSVC. In current literature, both terms are used according to individual preference. We prefer to use the term left azygos vein rather than hemiazygos to indicate the course and length of this vein.
Normal Development of the Portal Vein
Venous return of the primitive gut circulation is by way of the vitelline veins of the yolk sac that become confluent with the right and left omphalomesenteric veins, which enter the sinus venosus posteriorly (Fig. 36.4). As the omphalomesenteric veins approach the heart, they lie adjacent to the developing liver. The proximal portion of the omphalomesenteric veins breaks up into
a maze of small channels ramifying through the liver substance. The distal portion brings blood from the yolk sac and the intestine to the liver. When the yolk sac disappears and the intestine grows, the omphalic (yolk sac) portion of these veins also disappears. The mesenteric part persists and grows to match the length and complexity of the growing intestine (6). By the sixth week of gestation, the paired mesenteric veins have formed anastomoses with each other. A week later, the left mesenteric vein involutes cephalad to the anastomosis, while the right mesenteric vein involutes caudal to the anastomosis. This results in the unpaired hepatic portal vein that connects the veins of the intestine and the spleen with the circulation of the liver.
a maze of small channels ramifying through the liver substance. The distal portion brings blood from the yolk sac and the intestine to the liver. When the yolk sac disappears and the intestine grows, the omphalic (yolk sac) portion of these veins also disappears. The mesenteric part persists and grows to match the length and complexity of the growing intestine (6). By the sixth week of gestation, the paired mesenteric veins have formed anastomoses with each other. A week later, the left mesenteric vein involutes cephalad to the anastomosis, while the right mesenteric vein involutes caudal to the anastomosis. This results in the unpaired hepatic portal vein that connects the veins of the intestine and the spleen with the circulation of the liver.
Anomalies of the Superior Venae Cavae
Bilateral Superior Venae Cavae with Normal Drainage to the Right Atrium
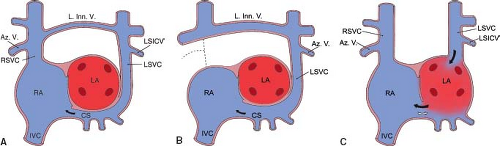
Persistence of the LSVC is thought to result from failure of the left anterior and left common cardinal veins to involute (5). Descriptions of persistent LSVC date back to 1787 (8). In 92% of cases, the LSVC drains into the RA through the coronary sinus (9). In the remainder, it drains into the LA by means of a partially or completely unroofed coronary sinus. The incidence of persistent LSVC in two large autopsy series was approximately 0.3% (10,11). Among patients with congenital heart disease, the prevalence of LSVC is much higher (12). Congenital heart malformations that show a high frequency of persistent LSVC are tetralogy of Fallot (11%), atrioventricular septal defects (19%), mitral atresia (17%) (13), and juxtaposition of the right atrial appendage (RAA) (34%) (14). Whereas persistent LSVC to coronary sinus results in normal return of systemic venous blood to the RA, this anomaly may have clinical implications for patients with associated cardiac malformations.
Anatomy
The size of the LSVC varies. It may be smaller than, equal to, or larger than the size of the RSVC. An LIV of variable size may be present in 60% of cases (15). The LSVC starts at the junction of the left jugular and left subclavian veins. It descends in front of the aortic arch and the left pulmonary vessels and, after receiving the left superior intercostal vein, it penetrates the pericardium. It then proceeds obliquely along the posterior wall of the LA and joins the coronary sinus in the posterior left atrioventricular groove (5,10). The flow of the LSVC blood into the coronary sinus results in its enlargement and posterior displacement of its orifice on the floor of the RA (Figs. 36.7 and 36.8). The thebesian valve seldom, if ever, is present.
Clinical Manifestations
When the LSVC drains into the RA through the coronary sinus, physiology is normal and there are no clinical manifestations. When this anomaly accompanies other congenital cardiac malformations, it may pose diagnostic and technical difficulties during catheterization and cardiac surgery (16). Enlargement of the coronary sinus resulting from a persistent LSVC may interfere with blood flow from the LA into the left ventricle. An increase in the magnitude of the left-to-right shunt at the atrial level was found in patients with secundum atrial septal defects (ASDs), persistent LSVC, and dilated coronary sinus (17,18).
Diagnostic Features
The presence of an LSVC can be suspected on a chest radiogram based on a shadow along the left upper border of the mediastinum. Echocardiography is the most widely used noninvasive method to detect an LSVC (19). Huhta et al. (20), before color Doppler became available, demonstrated that detection of LSVC by 2-D echocardiography had a specificity of 100% and a sensitivity of 96%.
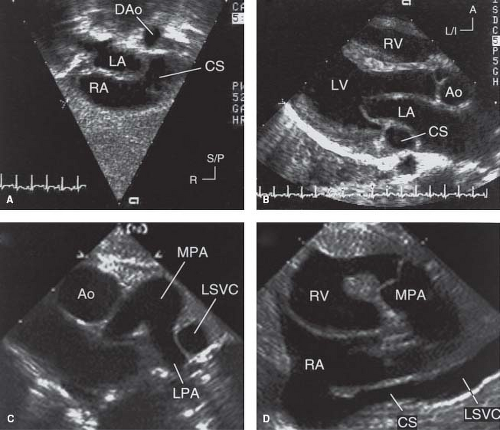
Imaging of a dilated coronary sinus is often the first clue to the diagnosis of an LSVC during the course of an echocardiographic examination. The coronary sinus can be imaged from the subcostal, apical, and parasternal windows (Fig. 36.9). It appears as a tubular structure in the posterior left atrioventricular groove with an opening into the posteroinferior aspect of the RA adjacent to the orifice of the IVC. The LSVC and its drainage into the coronary sinus can be imaged from the subcostal short-axis view in patients with adequate acoustic windows. In most patients, the LSVC can be imaged from the suprasternal notch or from the high left parasternal/subclavicular
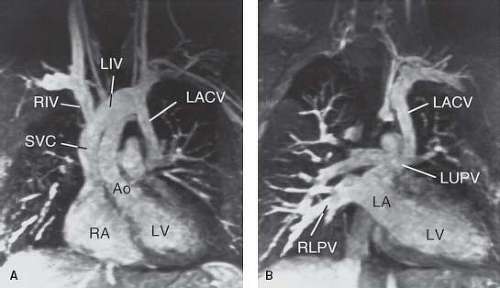
windows (Fig. 36.9D). From these windows, the presence and size of the LIV can be imaged. In general, there is an inverse relationship between the caliber of the LSVC and the LIV. Pulsed and color Doppler flow mapping are useful in demonstrating typical systemic venous flow patterns in the LSVC. Flow mapping by Doppler also is important in differentiating between an LSVC and other veins that may connect with the LIV. These include partial or total anomalous pulmonary venous connection, a left superior intercostal vein, and a levoatrialcardinal vein (LACV). In contrast to an LSVC to an intact coronary sinus, however, the direction of blood flow in these veins is expected to be into the LIV.
windows (Fig. 36.9D). From these windows, the presence and size of the LIV can be imaged. In general, there is an inverse relationship between the caliber of the LSVC and the LIV. Pulsed and color Doppler flow mapping are useful in demonstrating typical systemic venous flow patterns in the LSVC. Flow mapping by Doppler also is important in differentiating between an LSVC and other veins that may connect with the LIV. These include partial or total anomalous pulmonary venous connection, a left superior intercostal vein, and a levoatrialcardinal vein (LACV). In contrast to an LSVC to an intact coronary sinus, however, the direction of blood flow in these veins is expected to be into the LIV.
An anomalously connecting pulmonary vein can be diagnosed by following the vein into the lung hilum, by the Doppler flow pattern, and by the absence of normal connection of the involved pulmonary vein to the LA. The left superior intercostal vein is a small systemic vein that can be followed toward the anterior chest wall. An LACV is a remnant of an early embryonic venous channel that connects the splanchnic plexus of the lungs with the cardinal system. In the mature heart, it connects the LA or a pulmonary vein with the left innominate or other systemic veins (Fig. 36.10). Typically, it is associated with severe left atrial outlet obstruction, such as mitral stenosis or atresia with a restrictive patent foramen ovale or an intact atrial septum, and provides an alternative egress for pulmonary venous blood (21). The diagnosis can be established by following the anomalous vein from its origin (either from the LA or from one of the pulmonary veins) to its termination in a systemic vein. Unlike persistent LSVC that courses anterior to the left pulmonary artery (LPA), an LACV typically ascends posterior to it (22). The different positions of an LSVC and an LACV in relation to the LPA are important for distinguishing between these venous structures. Although in some patients the LACV can be ligated safely or closed in the catheterization laboratory, inadvertent obstruction to venous drainage from the left lung can occur. Demonstration of retrograde flow in an LSVC toward the LIV indicates that the LSVC is venting obstructed coronary sinus flow, usually owing to stenosis or atresia of the coronary sinus orifice.
Persistent LSVC can be diagnosed by magnetic resonance imaging (MRI) either by spin echo or by gradient echo sequences. Magnetic resonance angiography (MRA) is particularly suitable for rapid noninvasive delineation of systemic venous anatomy (Fig. 36.10). By cardiac catheterization, LSVC can be suspected by the presence of higher-than-expected coronary sinus oxygen saturation. LIV angiography with balloon occlusion proximal to the injection site is diagnostic. The LSVC can be approached either through the right SVC (when the innominate vein is present) or through the coronary sinus (Fig. 36.7). The diagnosis of systemic venous anomalies can be established reliably by echocardiography and MRI. Cardiac catheterization is unnecessary in most patients.
Treatment
No treatment is necessary for an isolated LSVC to an intact coronary sinus.
Bilateral Superior Venae Cavae with an Unroofed Coronary Sinus
Anatomy
Partial or complete absence of the common wall between the LA and the coronary sinus has been termed partial or complete unroofing of the coronary sinus. In hearts with partial or complete unroofing of the coronary sinus, a persistent LSVC drains into the LA. In patients with a normal septum primum and septum secundum, the orifice of the unroofed coronary sinus will function as an interatrial communication. This type of interatrial communication has been erroneously diagnosed as a posterior ASD occurring in association with a persistent LSVC and unroofed coronary sinus (23).
Bilateral SVC with an unroofed coronary sinus may occur in association with other congenital heart defects and rarely as an isolated lesion (24). Visceral heterotaxy with asplenia exhibits the highest incidence of bilateral SVCs with a completely unroofed coronary sinus. In a study of 58 postmortem cases of visceral heterotaxy with asplenia, the incidence was 67%, and in 46 postmortem cases of polysplenia, the incidence was 13% (1). The reason for the high frequency of an unroofed coronary sinus in asplenia is not
known. The high frequency of bilateral SVCs in asplenia probably represents a remnant of the normal early embryonic symmetry of the systemic veins that is characteristic of visceral heterotaxy.
known. The high frequency of bilateral SVCs in asplenia probably represents a remnant of the normal early embryonic symmetry of the systemic veins that is characteristic of visceral heterotaxy.
As mentioned, a persistent LSVC entering the LA when the coronary sinus is unroofed should be distinguished from persistence of an embryonic connection between the LA and a systemic vein (LACV) or between the left pulmonary veins and a systemic vein (vertical vein) (25). Almost all cases of LACV have been associated with severe left atrial outlet stenosis or atresia (21). Rarely, a normally connected left upper pulmonary vein to the LA may maintain the early embryonic connection with the LIV without left atrial outflow stenosis or atresia (21). We also encountered such a case (Fig. 36.10). It is interesting to note that both cases had coarctation of the aorta.
Clinical Manifestations
Most patients with an isolated persistent LSVC to a partially or completely unroofed coronary sinus also have a large coronary sinus ostium that functions as an interatrial communication (Raghib syndrome) (Fig. 36.8C). The hemodynamic consequences of the Raghib syndrome are cyanosis and left-to-right shunting through the “ASD.” Systemic arterial desaturation is caused by mixing of LSVC blood with pulmonary venous blood in the LA. The degree of arterial desaturation is related to the net right-to-left shunt, which, in turn, depends on the amount of systemic venous blood carried by the LSVC and the proportion of systemic venous blood that crosses the atrial septum and reaches the pulmonary circulation. In most patients, the arterial oxygen saturation ranges between 85% and 95%. These patients exhibit varying degrees of cyanosis, clubbing of the nail beds, and polycythemia. They are at risk for complications of right-to-left shunting, including paradoxical emboli, brain abscess, strokes, and death. In some patients, the coronary sinus ostium is atretic and there is no significant interatrial communication. The only clinical manifestations in these patients are cyanosis and its sequelae. Patients with a significant interatrial communication exhibit signs and symptoms related to left-to-right shunting as well as cyanosis. When a persistent LSVC to an unroofed coronary sinus is associated with complex congenital heart disease (often in the context of heterotaxy syndrome), the clinical features of LSVC drainage to the LA are obscured by manifestations of the associated anomalies. When right atrial outflow stenosis or atresia coexists with a persistent LSVC to an unroofed coronary sinus, the shunt is exclusively from right to left.
Diagnostic Features
The LSVC may appear as a shadow along the left upper border of the mediastinum on the chest radiogram. The electrocardiographic features of isolated LSVC to an unroofed coronary sinus are similar to those in patients with uncomplicated secundum ASD. The frontal axis of the P wave may be abnormal in patients with heterotaxy syndrome, reflecting a left sinoatrial node or an ectopic atrial rhythm.
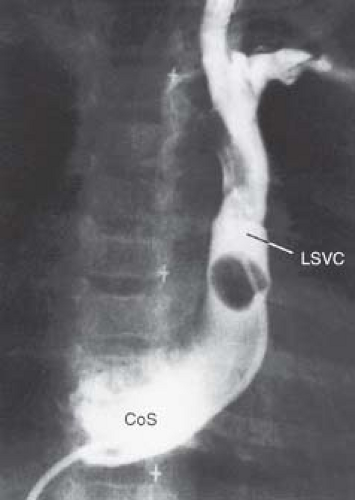
Echocardiography is the definitive imaging modality in most patients (26). The LSVC and its drainage into the LA can be imaged from the subcostal window in young patients and from the precordial and suprasternal notch windows in most patients. The posterior left atrioventricular groove is examined in detail to ascertain the extent of deficiency of the coronary sinus septum. When the coronary sinus septum is completely unroofed, the LSVC terminates in the upper left posterior corner of the LA between the left upper pulmonary vein posteriorly and the left atrial appendage anteriorly. Flow mapping with color Doppler is useful in demonstrating flow from the LSVC into the LA. If the diagnosis is still in doubt, a contrast injection in a left arm vein establishes the diagnosis by demonstration of microbubbles in the LA before they appear in the RA. MRI is increasingly used to establish the diagnosis, especially when technical limitations compromise the quality of echocardiography or when the anatomy has not been completely delineated. By cardiac catheterization, the diagnosis is established by demonstration of a step-down in oxygen saturation between the pulmonary veins and the LA and by LSVC-selective angiocardiography (Fig. 36.11).
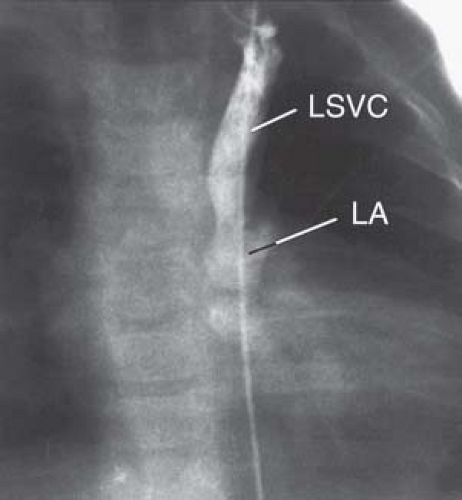 Figure 36.11 Venogram of a left superior vena cava (LSVC) entering the left side of a common atrium via an unroofed coronary sinus in a 15-month-old boy with visceral heterotaxy. A catheter inserted into the right femoral vein entered a left venous channel that crossed the diaphragm and entered the left side of a common atrium. LA, left atrium. The additional venous anomalies of this case are presented in Figure 36.20. (The angiocardiogram of this patient was kindly provided by Dr. John Murphy, duPont Hospital for Children, Wilmington, DE.) |
Treatment
The coronary sinus defect of a partially or completely unroofed coronary sinus that receives an LSVC must be repaired to avoid complications of cyanosis and its sequelae and of chronic left-to-right shunting. Accurate preoperative diagnosis is essential to avoid inadvertent closure of an enlarged coronary sinus orifice that is mistaken for a secundum ASD. Such an error will result in significant postoperative cyanosis.
If the LSVC is relatively small and there is an adequate-sized LIV, the LSVC can be ligated and the interatrial communication closed, leaving the coronary sinus blood to drain into the LA. In the absence of an adequate-sized bridging LIV, the coronary sinus is “reroofed” (27). This is achieved by baffling the LSVC along the posterior wall of the LA into the RA. Care is taken to avoid the orifices of the pulmonary veins. A coronary sinus defect also can be closed in the catheterization laboratory with an ASD device.
Absent Right Superior Vena Cava in Visceroatrial Situs Solitus
Anatomy
Absent or atretic right SVC in visceroatrial situs solitus is rare, occurring in 0.07% to 0.13% of patients with cardiovascular malformations (28,29). In a study of 121 cases, we found that this anomaly occurred both in patients with structurally normal hearts (54%) and in patients with congenital heart defects (46%) (30). This anomaly is characterized by persistence of the LSVC draining to the RA via the coronary sinus and by left-sided azygos vein draining into the LSVC (Fig. 36.8B). Less constant features
were additional cardiovascular malformations (46%) and rhythm abnormalities (35%) that usually appeared related to complications of old age. In seven cases (6%), the coronary sinus was unroofed; hence, the LSVC drained into the LA (30). The LSVC, in cases of atretic or absent right SVC, should not be considered an inverted SVC. The origin and course of this LSVC are exactly the same as the origin and course of the LSVC, which may be present when the right SVC is normal (30).
were additional cardiovascular malformations (46%) and rhythm abnormalities (35%) that usually appeared related to complications of old age. In seven cases (6%), the coronary sinus was unroofed; hence, the LSVC drained into the LA (30). The LSVC, in cases of atretic or absent right SVC, should not be considered an inverted SVC. The origin and course of this LSVC are exactly the same as the origin and course of the LSVC, which may be present when the right SVC is normal (30).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree