Chapter 45 Abdominal Wall, Umbilicus, Peritoneum, Mesenteries, Omentum, and Retroperitoneum
Abdominal Wall And Umbilicus
Anatomy
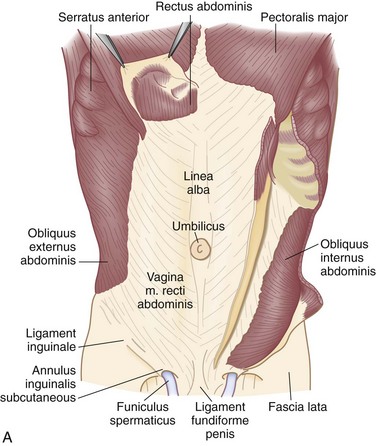
There are nine layers to the abdominal wall—skin, subcutaneous tissue, superficial fascia, external oblique muscle, internal oblique muscle, transversus abdominis muscle, transversalis fascia, preperitoneal adipose and areolar tissue, and peritoneum (Fig. 45-1).
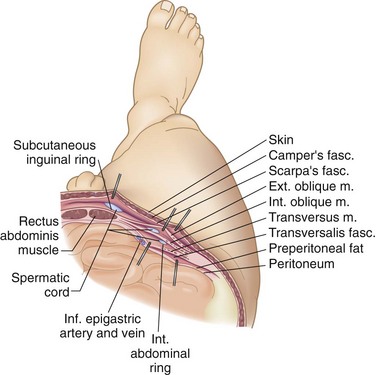
FIGURE 45-1 The nine layers of the anterolateral abdominal wall.
(From Thorek P: Anatomy in surgery, ed 2, Philadelphia, 1962, JB Lippincott, p 358.)
Muscle and Investing Fascias
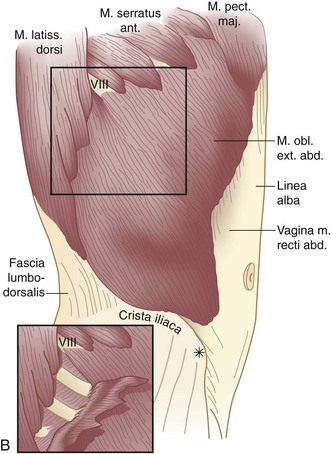
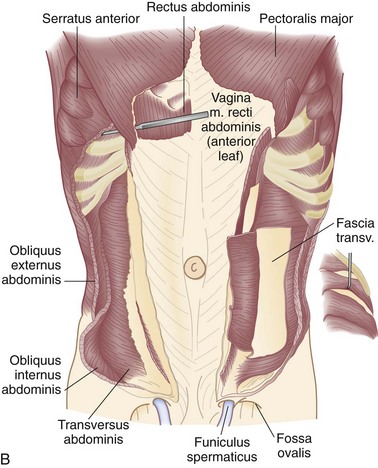
The muscles of the anterolateral abdominal wall include the external and internal oblique and transversus abdominis. These flat muscles enclose much of the circumference of the torso and give rise anteriorly to a broad flat aponeurosis investing the rectus abdominis muscles, termed the rectus sheath. The external oblique muscles are the largest and thickest of the flat abdominal wall muscles. They originate from the lower seven ribs and course in a superolateral to inferomedial direction. The most posterior of the fibers run vertically downward to insert into the anterior half of the iliac crest. At the midclavicular line, the muscle fibers give rise to a flat strong aponeurosis that passes anteriorly to the rectus sheath to insert medially into the linea alba (Fig. 45-2). The lower portion of the external oblique aponeurosis is rolled posteriorly and superiorly on itself to form a groove on which the spermatic cord lies. This portion of the external oblique aponeurosis extends from the anterior superior iliac spine to the pubic tubercle and is termed the inguinal or Poupart’s ligament. The inguinal ligament is the lower free edge of the external oblique aponeurosis posterior to which pass the femoral artery, vein, and nerve and the iliacus, psoas major, and pectineus muscles. A femoral hernia passes posterior to the inguinal ligament, whereas an inguinal hernia passes anterior and superior to this ligament. The shelving edge of the inguinal ligament is used in various repairs of inguinal hernia, including the Bassini and the Lichtenstein tension-free repair (see Chapter 46).
The internal oblique muscle originates from the iliopsoas fascia beneath the lateral half of the inguinal ligament, from the anterior two thirds of the iliac crest and lumbodorsal fascia. Its fibers course in a direction opposite to those of the external oblique—that is, inferolateral to superomedial. The uppermost fibers insert into the lower five ribs and their cartilages (Fig. 45-3; see Fig. 45-2A). The central fibers form an aponeurosis at the semilunar line, which, above the semicircular line (of Douglas), is divided into anterior and posterior lamellae that envelop the rectus abdominis muscle. Below the semicircular line, the aponeurosis of the internal oblique muscle courses anteriorly to the rectus abdominis muscle as part of the anterior rectus sheath. The lowermost fibers of the internal oblique muscle pursue an inferomedial course, paralleling that of the spermatic cord, to insert between the symphysis pubis and pubic tubercle. Some of the lower muscle fascicles accompany the spermatic cord into the scrotum as the cremasteric muscle.
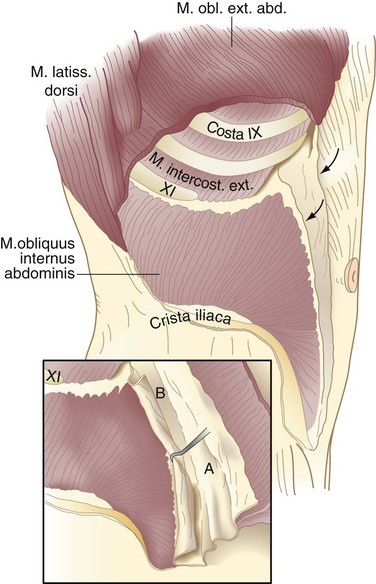
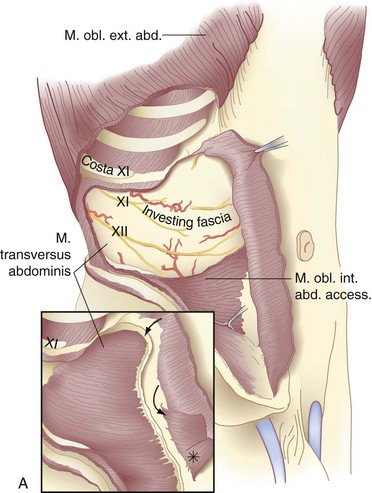
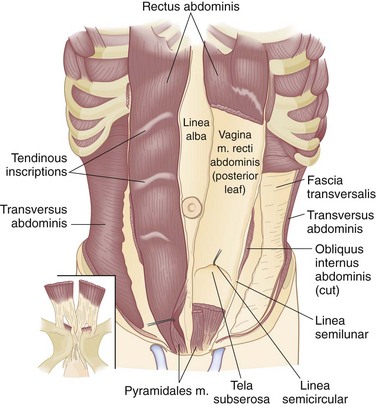
The transversus abdominis muscle is the smallest of the muscles of the anterolateral abdominal wall. It arises from the lower six costal cartilages, spines of the lumbar vertebra, iliac crest, and iliopsoas fascia beneath the lateral third of the inguinal ligament. The fibers course transversely to give rise to a flat aponeurotic sheet that passes posterior to the rectus abdominis muscle above the semicircular line and anterior to the muscle below it (Fig. 45-4). The inferiormost fibers of the transversus abdominis originating from the iliopsoas fascia pass inferomedially along with the lower fibers of the internal oblique muscle. These fibers form the aponeurotic arch of the transversus abdominis muscle, which lies superior to Hesselbach’s triangle and is an important anatomic landmark in the repair of inguinal hernias, particularly Bassini’s operation and Cooper’s ligament repairs. Hesselbach’s triangle is the site of direct inguinal hernias and is bordered by the inguinal ligament inferiorly, lateral margin of the rectus sheath medially, and inferior epigastric vessels laterally. The floor of this triangle is composed of transversalis fascia.
The transversalis fascia covers the deep surface of the transversus abdominis muscle and, with its various extensions, forms a complete fascial envelope around the abdominal cavity (Fig. 45-5; see Fig. 45-4B). This fascial layer is regionally named for the muscles that it covers—for example, the iliopsoas fascia, obturator fascia, and inferior fascia of the respiratory diaphragm. The transversalis fascia binds together the muscle and aponeurotic fascicles into a continuous layer and reinforces weak areas where the aponeurotic fibers are sparse. This layer is responsible for the structural integrity of the abdominal wall and, by definition, a hernia results from a defect in the transversalis fascia.
The rectus abdominis muscles are paired muscles that appear as long, flat triangular ribbons wider at their origin on the anterior surfaces of the fifth, sixth, and seventh costal cartilages and the xiphoid process than at their insertion on the pubic crest and pubic symphysis. Each muscle is composed of long parallel fascicles interrupted by three to five tendinous inscriptions (Fig. 45-5), which attach the rectus abdominis muscle to the anterior rectus sheath. There is no similar attachment to the posterior rectus sheath. These muscles lie adjacent to each other, separated only by the linea alba. In addition to supporting the abdominal wall and protecting its contents, contraction of these powerful muscles flexes the vertebral column.
Preperitoneal Space and Peritoneum
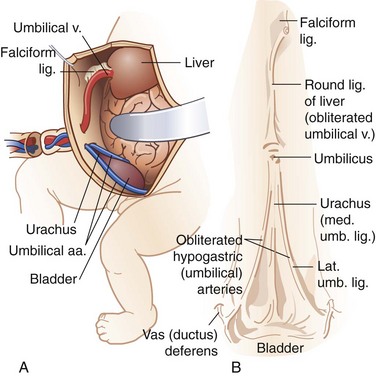
The round ligament, or ligamentum teres, is contained within the free margin of the falciform ligament and represents the obliterated umbilical vein, coursing from the umbilicus to the left branch of the portal vein (Fig. 45-6). The parietal peritoneum is the innermost layer of the abdominal wall. It consists of a thin layer of dense, irregular connective tissue covered on its inner surface by a single layer of squamous mesothelium.
Vessels and Nerves of the Abdominal Wall
Vascular Supply
The anterolateral abdominal wall receives its arterial supply from the last six intercostals and four lumbar arteries, superior and inferior epigastric arteries, and deep circumflex iliac arteries (Fig. 45-7). The trunks of the intercostal and lumbar arteries, together with the intercostal, iliohypogastric, and ilioinguinal nerves, course between the transversus abdominis and internal oblique muscles. The distalmost extensions of these vessels pierce the lateral margins of the rectus sheath at various levels and communicate freely with branches of the superior and inferior epigastric arteries. The superior epigastric artery, one of the terminal branches of the internal mammary artery, reaches the posterior surface of the rectus abdominis muscle through the costoxiphoid space in the diaphragm. It descends within the rectus sheath to anastomose with branches of the inferior epigastric artery. The inferior epigastric artery, derived from the external iliac artery just proximal to the inguinal ligament, courses through the preperitoneal areolar tissue to enter the lateral rectus sheath at the semilunar line of Douglas. The deep circumflex iliac artery, arising from the lateral aspect of the external iliac artery near the origin of the inferior epigastric artery, gives rise to an ascending branch, which penetrates the abdominal wall musculature just above the iliac crest, near the anterior superior iliac spine.
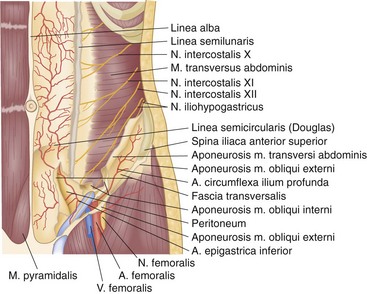
FIGURE 45-7 Arteries and nerves of the anterolateral abdominal wall.
(From McVay C: Anson and McVay’s surgical anatomy, ed 6, Philadelphia, 1984, WB Saunders, p 501.)
The venous drainage of the anterior abdominal wall follows a relatively simple pattern in which the superficial veins above the umbilicus empty into the superior vena cava by way of the internal mammary, intercostal, and long thoracic veins. The veins inferior to the umbilicus—the superficial epigastric, circumflex iliac, and pudendal veins—converge toward the saphenous opening in the groin to enter the saphenous vein and become a tributary to the inferior vena cava (Fig. 45-8). The numerous anastomoses between the infraumbilical and supraumbilical venous systems provide collateral pathways whereby venous return to the heart may bypass an obstruction of the superior or inferior vena cava. The paraumbilical vein, which passes from the left branch of the portal vein along the ligamentum teres to the umbilicus, provides important communication between the veins of the superficial abdominal wall and portal system in patients with portal venous obstruction. In this setting, portal blood flow is diverted away from the higher pressure portal system through the paraumbilical veins to the lower pressure veins of the anterior abdominal wall. The dilated superficial paraumbilical veins in this setting are termed caput medusae.
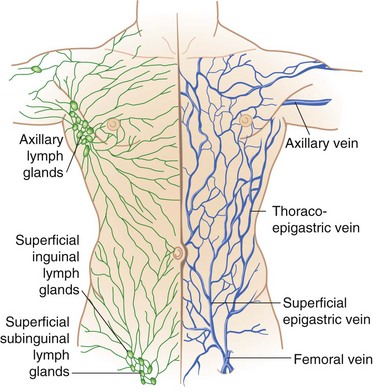
FIGURE 45-8 Venous and lymphatic drainage of the anterolateral abdominal wall.
(From Thorek P: Anatomy in surgery, ed 2, Philadelphia, 1962, JB Lippincott, p 345.)
Innervation
The anterior rami of the thoracic nerves follow a curvilinear course forward in the intercostal spaces toward the midline of the body (see Fig. 45-7). The upper six thoracic nerves end near the sternum as anterior cutaneous sensory branches. Thoracic nerves 7 to 12 pass behind the costal cartilages and lower ribs to enter a plane between the internal oblique muscle and the transversus abdominis. The seventh and eighth nerves course slightly upward or horizontally to reach the epigastrium, whereas the lower nerves have an increasingly caudal trajectory. As these nerves course medially, they provide motor branches to the abdominal wall musculature. Medially, they perforate the rectus sheath to provide sensory innervation to the anterior abdominal wall. The anterior ramus of the 10th thoracic nerve reaches the skin at the level of the umbilicus and the 12th thoracic nerve innervates the skin of the hypogastrium.
Abnormalities of the Abdominal Wall
These can be congenital or acquired.
Congenital Abnormalities
Umbilical Hernias
Umbilical hernias may be classified into three distinct forms:
Gastroschisis
Gastroschisis is another congenital defect of the abdominal wall in which the umbilical membrane has ruptured in utero, allowing the intestine to herniate outside the abdominal cavity. The defect is almost always to the right of the umbilical cord and the intestine is not covered with skin or amnion. Typically, the intestine has not undergone complete mesenteric rotation and fixation; hence, the infant is at risk for mesenteric volvulus, with resultant intestinal ischemia and necrosis. Concomitant congenital anomalies occur in about 10% of these patients. Both omphalocele and gastroschisis are discussed in greater detail in Chapter 67.
Infantile Umbilical Hernia
Infantile umbilical hernias appear within a few days or weeks after the stump of the umbilical cord has sloughed. It is caused by a weakness in the adhesion between the scarred remnants of the umbilical cord and umbilical ring. In contrast to omphalocele, the infantile umbilical hernia is covered by skin. Generally, these small hernias occur in the superior margin of the umbilical ring. They are easily reducible and become prominent when the infant cries. Most of these hernias resolve within the first 24 months of life, and complications such as strangulation are rare. Operative repair is indicated for those children in whom the hernia persists beyond the age of 3 or 4 years. This condition and its management are discussed further in Chapters 46 and 67.
Abnormalities Resulting from Persistence of the Omphalomesenteric Duct
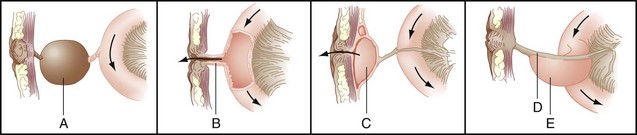
During fetal development, the midgut communicates widely with the yolk sac through the vitelline or omphalomesenteric duct. As the abdominal wall components approximate one another, the omphalomesenteric duct narrows and comes to lie within the umbilical cord. Over time, communication between the yolk sac and intestine becomes obliterated and the intestine resides free within the peritoneal cavity. Persistence of part or all of the omphalomesenteric duct results in a variety of abnormalities related to the intestine and abdominal wall (Fig. 45-9).
Persistence of the intestinal end of the omphalomesenteric duct results in Meckel’s diverticulum. These true diverticula arise from the antimesenteric border of the small intestine, most often the ileum. A Rule of 2s is often applied to these lesions in that they are found in approximately 2% of the population, are within 2 feet of the ileocecal valve, are often 2 inches in length, and contain two types of ectopic mucosa (gastric and pancreatic). Meckel’s diverticula may be complicated by inflammation, perforation, hemorrhage, or obstruction. GI bleeding is caused by peptic ulceration of adjacent intestinal mucosa from hydrochloric acid secreted by ectopic parietal cells within the diverticulum. Intestinal obstruction associated with Meckel’s diverticulum is usually caused by intussusception or volvulus around an abnormal fibrous connection between the diverticulum and posterior aspect of the umbilicus. These lesions are discussed in Chapter 50.
Acquired Abnormalities
Anterior Abdominal Wall Hernias
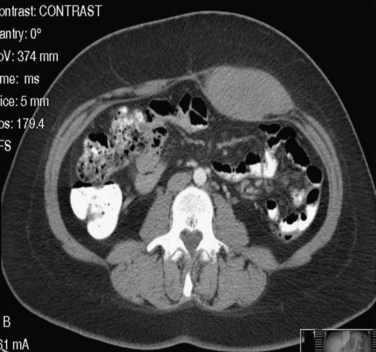
Epigastric hernias occur at sites through which vessels and nerves perforate the linea alba to course into the subcutaneum. Through these openings, extraperitoneal areolar tissue and, at times, peritoneum may herniate into the subcutaneous tissue. Although these hernias are often small, they may produce significant localized pain and tenderness because of direct pressure of the hernia sac and its contents on the nerves emerging through the same fascial opening. Spigelian hernias occur through the fascia in the region of the semilunar line and present with localized pain and tenderness. The hernia sac is only rarely palpable because it is often small and tends to remain beneath the external oblique aponeurosis. Ultrasonography of the abdominal wall or computed tomography (CT) with thin cuts through the abdomen, after careful marking of the suspected site, should be diagnostic. Treatment consists of simple operative closure of the fascial defect. These hernias are discussed in Chapter 46.
Rectus Sheath Hematoma
Rectus sheath hematoma is an uncommon condition characterized by acute abdominal pain and the appearance of an abdominal wall mass. It is more common in women than men and in older than younger individuals. A review of 126 patients with rectus sheath hematomas treated at the Mayo Clinic found that almost 70% were receiving anticoagulants at the time of diagnosis. A history of nonsurgical abdominal wall trauma or injury is common (48%), as is the presence of a cough (29%).1 In young women, rectus sheath hematomas have been associated with pregnancy.
Patients with rectus sheath hematomas usually present with the sudden onset of abdominal pain, which may be severe and is often exacerbated by movements requiring contraction of the abdominal wall. Physical examination will demonstrate tenderness over the rectus sheath, often with voluntary guarding. An abdominal wall mass may be noted in some patients, 63% in the Mayo Clinic series.1 Abdominal wall ecchymosis, including periumbilical ecchymosis (Cullen’s sign) and blue discoloration in the flanks (Grey Turner’s sign), may be present if there is a delay from the onset of symptoms to presentation. The pain and tenderness associated with this process may be severe enough to suggest peritonitis. In those cases in which the hematoma expands into the perivesical and preperitoneal spaces, the hematocrit level may fall, although hemodynamic instability is uncommon.
Ultrasonography or CT will confirm the presence of the hematoma and localize it to the abdominal wall in almost all cases. Usually, these patients may be managed successfully with rest and analgesics and, if necessary, blood transfusion. In the Mayo Clinic series, almost 90% of patients were managed successfully in this manner.1 In general, coagulopathies are corrected, although continued anticoagulation of selected patients may be prudent, depending on the indications for anticoagulation and seriousness of the bleeding. Progression of the hematoma may necessitate angiographic embolization of the bleeding vessel or, uncommonly, operative evacuation of the hematoma and hemostasis.
Malignancies of the Abdominal Wall
Desmoid Tumor
The frequency of desmoid tumors in the general population is 2.4 to 4.3 cases/million; this risk increases 1000-fold in patients with FAP.2,3 The vast majority of desmoid tumors are sporadic, typically in young women during pregnancy or within a year of childbirth. Oral contraceptive use has also been associated with the occurrence of these tumors. These associations, combined with the detection of estrogen receptors within the tumor, suggest a regulatory role for estrogen in this disease.
Patients with a desmoid tumor present with an asymptomatic mass or with symptoms related to mass effect from the tumor. There is often a temporal association between the discovery of the tumor and an antecedent history of abdominal trauma or operation.3 Imaging (CT or MRI) is necessary to delineate the extent of tumor involvement fully, but otherwise there is no need to perform staging for metastatic disease. On CT, a desmoid tumor appears as a homogeneous mass arising from the soft tissue of the abdominal wall (Fig. 45-10). A desmoid tumor will appear as a homogeneous and isointense mass compared with muscle on T1-weighted MRI images, whereas T2-weighted images demonstrate greater heterogeneity and a signal slightly less intense than fat.
Resection of the tumor with a wide margin of normal tissue is currently considered the optimal treatment. Often, the extent of this resection will require abdominal wall reconstruction with local tissue flaps or mesh prostheses. The completeness of resection is an important prognostic factor; Stojadinovic and colleagues4 have reported that 68% of desmoids tumors resected with a positive margin recur within 5 years, compared with none of the tumors in which the resection margin was free of disease.
Abdominal wall desmoids are responsive to radiation therapy, although the treatment effect is slow and may be progressive over several years. Radiotherapy alone is an acceptable treatment option for patients with unresectable desmoid tumors or tumors for which resection will be associated with high morbidity risks or major functional loss. A retrospective review from the M.D. Anderson Cancer Center has reported 10-year recurrence rates of 38% for surgery alone (27% for those with negative margins), 25% for combined surgery and radiation, and 24% for radiation therapy alone.5 It was also concluded that radiation therapy can assuage the adverse effect of positive margins on local tumor recurrence. Similar large studies have reported local control rates of approximately 80% with radiotherapy alone, rates that are consistently equivalent or even superior to surgery alone.6
The detection of estrogen receptors on desmoids tumors, as well as the association with pregnancy and oral contraceptives, provide some support for the use of antiestrogens, such as tamoxifen. Clinical improvement has been reported in 43% of patients receiving antiestrogens, although the response rate varies among studies. Tumor responses to antiestrogens are slow in onset but often last for several years.7,8 Most reports of NSAID treatment use sulindac but indomethacin has also been used. A study using combination high-dose tamoxifen and sulindac recommended this regimen as initial treatment for FAP-associated desmoid tumors.9
Various cytotoxic chemotherapy regimens have been used in the treatment of patients with inoperable desmoids. Methotrexate with vinblastine, doxorubicin-based therapy, and ifosfamide-based regimens have been reported, with positive responses in 20% to 40% of patients.7,10 For desmoids with rapid growth, medical oncologists may recommend therapies typically used for sarcomas, such as doxorubicin and dacarbazine. Recent reports have also suggested imatinib, a tyrosine kinase inhibitor, as another effective treatment option for patients with these tumors.11
Metastatic Disease
Metastases to the abdominal wall may occur by direct seeding of the abdominal wall during biopsy or resection of an intra-abdominal malignancy or by hematogenous spread of an advanced tumor. The risk of tumor implantation at the port site after laparoscopic colon resection for adenocarcinoma is 0.9% and has been shown in randomized controlled trials to be no different than the risk of tumor recurrence in the wound after open colon resections.12 The most common tumors that metastasize to soft tissue are lung, colon, melanoma, and renal cell tumors. Although metastases to soft tissue are unusual, the abdominal wall is the site of such recurrence in approximately 20% of cases.13 Similar to desmoids tumors or sarcomas, metastases to the abdominal wall present as a painless mass. Immunohistochemistry staining of the tumor may allow specific identification of the type of primary tumor and facilitate differentiation from primary sarcomas of the abdominal wall. The Sister Mary Joseph nodule is often described and seldom seen but represents a palpable nodule in the region of the umbilicus representing metastatic abdominal or pelvic cancer.
Peritoneum And Peritoneal Cavity
Anatomy
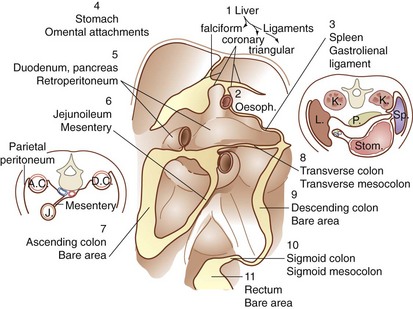
The peritoneal cavity is subdivided into interconnected compartments or spaces by 11 ligaments and mesenteries. The peritoneal ligaments or mesenteries include the coronary, gastrohepatic, hepatoduodenal, falciform, gastrocolic, duodenocolic, gastrosplenic, splenorenal, and phrenicocolic ligaments and the transverse mesocolon and small bowel mesentery (Fig. 45-11). These structures partition the abdomen into nine potential spaces—right and left subphrenic, subhepatic, supramesenteric and inframesenteric, right and left paracolic gutters, pelvis, and lesser space. These ligaments, mesenteries, and peritoneal spaces direct the circulation of fluid in the peritoneal cavity and thus may be useful in predicting the route of spread of infectious and malignant diseases. For example, perforation of the duodenum from peptic ulcer disease may result in the movement of fluid (and the development of abscesses) in the subhepatic space, right paracolic gutter, and pelvis. The blood supply to the visceral peritoneum is derived from the splanchnic blood vessels, whereas the parietal peritoneum is supplied by branches of the intercostals, subcostal, lumbar, and iliac vessels. The innervation of the visceral and parietal peritoneum is discussed earlier.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree