Fig. 17.1
Zones of the retroperitoneum. Zone 1 is a midline or central zone that is subcategorized into supramesocolic and inframesocolic regions. It extends from the diaphragm to the sacral promontory. Zone 2 is the lateral zone. An injury in this region results in hematomas associated with renal vascular or renal injuries. Zone 3 is the pelvic zone. Hematomas in the pelvis suggest injury to the iliac vessels. Zone 4 is referred to as the retrohepatic zone and suggests injury to the retrohepatic IVC and hepatic veins (not illustrated)
This zonal division of retroperitoneum aides in predicting the potentially injured structures and conceptualizing the management plan. Note that we here only discuss retroperitoneal zonal structures since that is where is the major vasculature is located.
Zone 1
This is a midline or central zone that is subcategorized into supramesocolic and inframesocolic regions. It extends from the diaphragm to the sacral promontory. The major vascular structures implicated in a central hematoma include the aorta and its major visceral braches, the portal vein, the subhepatic IVC and its major tributaries, the SMV, IMV, and renal veins.
Zone 2
This is the lateral zone. An injury in this region results in hematomas associated with renal vascular or renal injuries. It contains the adrenal glands, ureters, renal hilum, and parenchyma.
Zone 3
This is the pelvic zone. Hematomas in the pelvis suggest injury to the iliac vessels.
Zone 4
17.2.1 Anatomy
17.2.1.1 Inferior Vena Cava
The confluence of common iliac veins at the level of L5 marks the origin of IVC just posterior to the right common iliac artery (Fig. 17.2). It ascends along the right border of the vertebral bodies as it traverses through the diaphragmatic hiatus to drain into the right atrium. It receives numerous tributaries along its path along with a rich network of collaterals in the region of its bifurcation. The intra-abdominal IVC is commonly classified into four sections, namely, infrarenal, juxtarenal/perirenal, suprarenal/subhepatic, and the retrohepatic IVC.
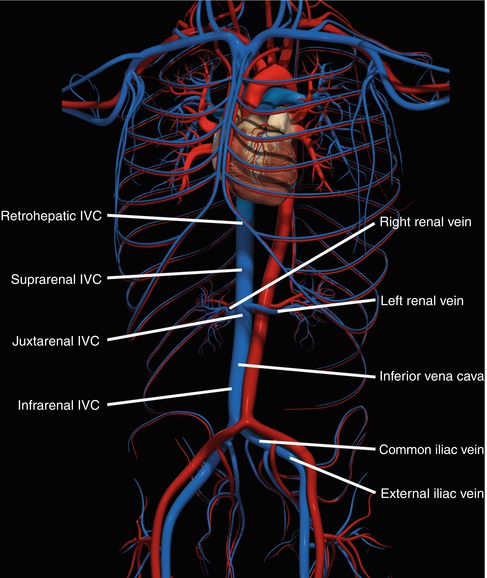

Fig. 17.2
The four zones of the inferior vena cava and major branches are illustrated
Juxtarenal IVC injuries involve the region from the level of renal veins to the inferior border of the liver and are located just posterior to the duodenum and pancreas. The short suprarenal/subhepatic segment of IVC extends in-between the juxtarenal and retrohepatic sections. Achieving control of injury in this region is technically challenging owing to its anatomical relations with the portal vein lying anteromedially, renal vessels posteriorly, and its close proximity to the liver superiorly.
The retrohepatic IVC is defined as inferior to the phrenic veins, superior to the right adrenal vein, and running in a groove across the “bare area of the liver” – uncovered, without capsule [14]. The unique aspect of the retrohepatic IVC is its complete confinement in hepatic suspensory ligaments, posteriorly by diaphragm and anteriorly by liver; making it difficult to explore.
17.2.1.2 Hepatic Veins
Three major hepatic veins drain the liver parenchyma into the IVC. The left, right, and middle hepatic veins enter the IVC before it traverses the diaphragm below the atriocaval junction. There is usually less than 1 cm of IVC between the hepatic veins and the diaphragm. In many patients, this is a purely theoretical space and should not be considered for clamping. The accessory hepatic veins also join the retrohepatic IVC just inferior to this confluence, all together binding the cava to the liver surface. An avulsion injury can completely uproot the hepatic veins resulting in multiple lethal lacerations involving the cava and hepatic veins.
17.2.1.3 Renal Veins
One of the special aspects of the left renal vein is its contribution to the cardinal (collateral) network and that it is approximately three times longer than the right. It receives drainage from left gonadal, adrenal, and a lumbar vein before draining into the IVC, along with a connective channel to the azygous system [15, 16]. This collateral network enables left renal vein ligation without a nephrectomy. The right renal vein, on the other hand, lacks such collaterals.
17.3 Presentation and Patterns of Injury
17.3.1 Mechanisms of Injury
Injury to the IVC represents 30–40 % of all intra-abdominal vascular injuries and is associated with a 50 % mortality rate. It occurs in 0.5–5 % of penetrating and in 0.6–1 % of the blunt abdominal traumatic events [17, 18]. Penetrating injuries to the IVC, like gunshot or stab wounds, often involve other organ systems. In rare scenarios, complete transection of both IVC and aorta can result in an aortocaval fistula [19]. Blunt injuries resulting from shearing deceleration forces can tear off vascular structures from their pedicles, as seen with hepatic veins and retrohepatic cava (explained previously), and cause intraparenchymal laceration or compression or crush injuries as commonly seen with renal veins.
The mechanism of renal vein injury involves two possible patterns. One is due to the stretch exerted on the vessel during the decelerating forces of blunt trauma. This involves the left renal vein more commonly [16, 20]. The other form is secondary to compression against vertebral bodies that can result in thrombosis and possible renal vein stenosis [16, 20].
17.3.2 Presentation and Diagnosis
A patient with an IAVI may present with normal vital signs or present with hypotension that may or may not be responsive to resuscitation. Because the venous system is low pressure, a majority of venous injuries will have a surrounding hematoma and present as a normotensive patient. This usually is the case with back or flank wounds. Most IVC injuries respond to intravenous fluid resuscitation even in cases of hemodynamic compromise [21]. Those with contained hematoma may be normotensive at arrival. Rarely, IVC injury may be associated with a simultaneous aortic injury presenting as aortocaval fistula that manifests as wide pulse pressure, hematuria, and an abdominal bruit (Fig. 17.3) [11, 22].
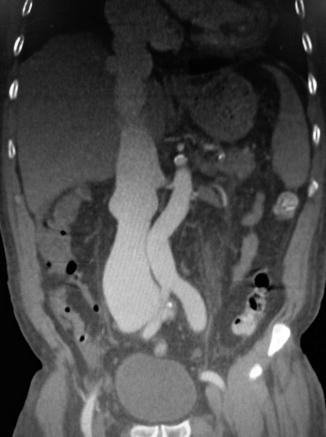

Fig. 17.3
Fistula between the common iliac artery and vein leading to significant distention of the inferior vena cava
Most high-energy gunshot wounds, massive blunt hepatic parenchymal fractures, or avulsion injuries can present as massive unremitting hemorrhage. Such patients frequently exsanguinate before reaching the operating room. All patients presenting with shock and penetrating injuries should be surgical explored after initial resuscitation with resuscitation continuing in the operating room.
Current imaging protocols are not designed to specifically evaluate the venous system in trauma patients. Radiographic evaluation may include abdominal x-ray and contrast CT scan to define the extent of the contained hematoma [23, 24]. Multiplanar reformatted images tend to be more intuitive as they can be tailored to allow visualization of the specific anatomic structures in nonaxial planes. In addition to the direct signs of venous injury that include thrombosis, avulsion, tear/rupture, extravasation, or pseudoaneurysm, one should be mindful of the more indirect signs of venous injury such as a perivascular hematoma, fat stranding, and vessel wall irregularity. Although intravenous pyelography is a sensitive tool in diagnosis of renal vascular injuries in cases with flank wounds or suspected renal trauma, CT has largely replaced it since it has the added advantage of diagnosing concurrent injuries. A CT scan demonstrating an early filling of the hepatic veins with contrast material in a trauma patient raises the suspicion for an associated arteriovenous fistula and should be followed with arteriography [25].
17.4 Management
17.4.1 General Principles
17.4.1.1 Blunt Trauma
Except for zone 1, blunt traumas with stable, nonpulsatile, non-expanding hematomas should not be disrupted. Traditionally, all zone 1 blunt hematomas are explored since packing is difficult in this region. It is estimated that as many as 40 % patients exsanguinate after surgical disruption of hematomas [11, 26, 27]. Zone 4 hematomas are often not directly visualized on laparotomy, but packing of the anterior liver is prudent. Persistent bleeding or suspicion of arterial injury is grounds for exploration in any zone.
Patients presenting after blunt injury who have hematuria and a perirenal retroperitoneal hematoma on imaging are managed with a nonoperative approach. Nonoperative management is successful in 95 % of these blunt renal trauma patients [26]. There is widespread consensus on nonoperative management of these injuries as renal function becomes severely impaired after 3–6 h of ischemia and salvage rates are only 25–35 % [16].
17.4.1.2 Penetrating Trauma
Retroperitoneal hematomas secondary to penetrating injury are usually best managed by surgical exploration in zones 1 and 3.
Zone 2 penetrating injuries may be selectively managed by not disrupting a stable hematoma. In hemodynamically normal patients, it is prudent to pack stable zone 2 hematomas and treat concomitant injuries such as bowel lacerations. If a zone 2 hematoma is not explored, it is essential to exclude an ischemic kidney and injuries to the renal pelvis or ureters. Use of additional imaging postoperatively may help define these types of injuries and whether the kidney is adequately perfused.
Penetrating retroperitoneal zone 4 injuries involving the IVC or hepatic veins are identified by persistent bleeding with dark blood despite the Pringle maneuver and liver packing. Such injuries are often fatal, but patients surviving to laparotomy will require exploration for bleeding control.
17.4.2 Operative Approach
17.4.2.1 Surgical Exposures
Operative preparation should include an adequate supply of cross-matched blood, large-bore supradiaphragmatic venous access, rolled packs, sponges, suctions, intravascular balloon occlusion catheters, 4-0 vascular sutures, and warming blankets to avoid hypothermia. The patient is positioned in slight reverse Trendelenburg position to avoid venous air embolism [28, 29]. In emergent laparotomy, patients should be prepped from chin to knees, to enable exposure to chest, groin, or for saphenous vein harvesting as autologous conduit.
Explore the abdomen via a long midline incision, extending from xiphoid process to pubic symphysis. The surgeon should expect distortion of normal anatomy due to significant displacement by a large retroperitoneal hematoma. All bleeding sites should be controlled with manual pressure, resection, or ligation of peripheral vessels, and all quadrants should be packed using laparotomy pads. The retroperitoneum is then evaluated for any IAVI or active bleeding.
Supramesocolic zone 1 hematomas require exploration by performing left medial visceral rotation that requires transection of the avascular line of Toldt in the left colon, incision of the lienosplenic ligament, and rotation of the colon, spleen, tail, and body of the pancreas as well as the stomach medially. This provides access to abdominal aorta and visceral vessels along with the left renal vascular pedicle. Alternatively, for juxta- and infrarenal IVC injuries, extended Kocher or Cattell-Braasch maneuver can be performed that involves transection along the avascular line of Toldt in the right colon and medial mobilization of the right colon, hepatic flexure, duodenum, and head of the pancreas. This exposes the superior mesenteric vessels and the infrahepatic/suprarenal IVC after incising the retroperitoneal tissue. The disadvantage of this maneuver is that there is a limited exposure to injuries at or above the level of supraceliac aorta and the hiatus. Venous hemorrhage can be controlled by finger or sponge-stick compression for proximal and distal control. Without imaging, the difficulty in zone 1 injuries is ascertaining whether left or right medial visceral rotation will give the best exposure. In general, right medial visceral rotation is best for IVC injuries, and left medial visceral rotation is best for aortic and other retroperitoneal zone 1 arterial injuries.
Exposure to the inframesocolic zone 1 hematomas involves cephalad displacement of the transverse colon and mesocolon, reflection of the small bowel to the right to locate the ligament of Treitz, and transecting it alongside the abdominal aorta until the left renal vein is located and the infrarenal aorta is exposed. The infrarenal IVC is exposed by transecting the avascular line of Toldt in the right colon along with a Kocher maneuver. Then the pancreas and duodenum are reflected to the left, and the retroperitoneal tissue covering the inferior vena cava is incised.
Exposure of the right/left lateral or zone 2 hematomas can be technically demanding. If the perirenal hematoma or active bleeding is located medially or if there is an expanding hematoma, vascular control at the level of the pedicle is advisable. It is performed by mobilization of the right colon and hepatic flexure, performing a Kocher maneuver thereby exposing the infrarenal inferior vena cava. The dissection is continued cephalad incising the tissues over the suprarenal infrahepatic inferior vena cava until the right renal vein is encountered. Similarly, for the left-sided exposure, the left colon and splenic flexure are mobilized, and the small bowel is reflected to the right. The ligament of Treitz is localized followed by cephalad mobilization of the transverse colon and mesocolon. The dissection is continued cephalad until the left renal vein is encountered over the abdominal aorta. Alternatively, the lateral aspects of Gerota’s fascia can be incised along with lifting the kidney up and medially to locate the hemorrhage if a perirenal hematoma or bleeding is localized laterally.
Exposure to the zone 4 or retrohepatic cava and major hepatic veins requires a right thoracoabdominal incision or a median sternotomy and mobilization of the right triangular ligament along with the caval crossing point at the level of right adrenal vein [5]. For left-sided exposure, a full-length incision of the left triangular ligament is performed. Another approach is through bilateral subcostal incision with upper midline vertical extension, to the left of the xiphoid process, commonly used in liver transplantation, has been described in pediatric cases to gain exposure to juxtahepatic vasculature [30]. Evans et al. describe that this type of incision provides adequate exposure to repair associated splenic, pancreatic, renal, or intestinal injury. The tethering of the retrohepatic IVC to the liver surface by its numerous tributaries mandates extensive division of liver along the lobar planes to access this segment of IVC. Radical hepatic mobilization and division further compounds the odds of fatality associated with retrohepatic cava and major hepatic venous trauma. Thus, this procedure is not recommended unless there is active bleeding that is not contained with perihepatic packing [5].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


