Abdominal Aortic Aneurysms
John E. Rectenwald
David M. Williams
James C. Stanley
Gilbert R. Upchurch Jr.
Abdominal aortic aneurysms represent a serious vascular disease that is the 14th most common cause of death in the United States. Abdominal aortic aneurysms (AAAs) develop in 3% to 9% of the population, and rupture of AAAs accounts for nearly 15,000 deaths each year in the United States (1). The infrarenal aorta is the site of 80% of all aortic aneurysms (2,3). Encounters with this disease will become even more frequent as society ages dramatically during the early decades of the 21st century.
USUAL CAUSES
AAAs are characterized by marked inflammation and an imbalance between the production and degradation of structural extracellular matrix proteins (4). Disruption and degradation of medial elastin and collagen are particularly prominent features of AAA formation. In this regard, increased local production of enzymes that degrade elastin and collagen—the matrix metalloproteinases—has been proposed as pivotal in vessel-wall degradation and in clinical progression of aneurysmal disease (5,6,7,8,9,10,11,12,13,14). Arteriosclerosis, a common finding in AAAs, compromises the structure of the aortic wall but is believed to be a secondary, not a primary, etiologic factor in AAA development. Rarer causes of AAAs include cystic medial necrosis, trauma, dissections, vasculitis, collagen diseases such as Marfan syndrome, and infection (Table 28.1).
Genetic factors appear important in AAA development; 15% of patients have a first-degree relative with an AAA (15). To date, no single gene mutation or protein deficiency has been associated with the common infrarenal AAA. However, a decrease in aortic wall type III collagen has been noted in individuals who have a first-degree relative with an AAA, in comparison with those without this family history of AAAs (16). Increases in the frequency of the Hp 2-1 haptoglobin phenotype, as well as the Kellpositive and MN blood groups, have also been noted in patients with AAAs. In contrast, a decrease in the incidence of AAAs is found in patients with type A Rh-negative blood group (17). In addition, one study suggests that a polymorphic alteration in the human lymphocyte antigen-DR B1 is important in the development of inflammatory AAAs (18). A DNA linkage analysis of 233 families with at least two individuals diagnosed with AAA identified two chromosomal regions (chromosomes 19g13 and 4q31) that may be correlated with AAA inheritance (19). In the next few years, genetic analyses are likely to reveal a number of contributing factors in individuals predisposed to the development of AAAs.
PRESENTING SYMPTOMS AND SIGNS
Most AAAs are asymptomatic. Palpation of the lateral borders of the aorta between the examiner’s fingertips on abdominal examination may reveal the existence of an AAA. However, prominent anterior pulsations alone are more likely to be caused by an ectatic, nonaneurysmal aorta. The aortic bifurcation is at the level of the umbilicus, and it is often difficult to palpate the infrarenal aorta in the epigastrium. In most patients,
physical examination alone is predictably unreliable in detecting AAA. In one series, AAAs were overlooked by physical examination in 62% of patients with known AAAs (20). Physical examination alone as a diagnostic maneuver is clearly not sensitive enough to exclude the diagnosis of an AAA.
physical examination alone is predictably unreliable in detecting AAA. In one series, AAAs were overlooked by physical examination in 62% of patients with known AAAs (20). Physical examination alone as a diagnostic maneuver is clearly not sensitive enough to exclude the diagnosis of an AAA.
TABLE 28.1. Various causes of abdominal aortic aneurysms | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Patients with AAAs may also have aneurysms of the femoral or popliteal arteries that are apparent on physical examination. A study from the University of Michigan of 251 patients with AAAs documented a 14% incidence of aneurysms of the femoral and popliteal arteries, all occurring in male patients (21). The presence of these extremity aneurysms may serve as a clue to the existence of an AAA. It is important to recognize that patients with femoral and popliteal artery aneurysms have an 85% and a 62% chance of having an AAA, respectively (22,23).
In contrast to an intact AAA, a ruptured AAA is always symptomatic. Unfortunately, the classic triad of acute hypotension, back or abdominal pain, and a palpable abdominal mass is an all-too-often inconsistent finding in this setting. A high index of suspicion is mandatory for making a timely diagnosis of these life-threatening lesions. In stable patients, the use of rapid abdominal ultrasonography (US) or computed tomography (CT) has gained popularity in many emergency departments, because both methods confirm the suspected diagnosis of a ruptured AAA, require no transport time, and are quite sensitive. However, any patient with hypotension and abdominal or back pain with a suspected expanding or ruptured AAA should be transported urgently to the operating room. Symptomatic AAAs represent true surgical emergencies and justify immediate operative intervention, without extensive time-consuming diagnostic studies.
HELPFUL TESTS
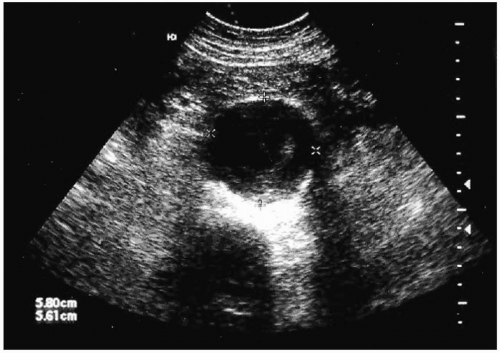
Once an AAA is suspected, a logical diagnostic algorithm should be followed to confirm the diagnosis (Fig. 28.1). Plain abdominal or lumbosacral radiographs performed in either the anteroposterior (AP) or lateral projections may suggest the presence of an AAA by demonstrating a rim of calcification in the outer aortic wall. The most useful means of establishing the diagnosis of an AAA is duplex US, a noninvasive, inexpensive test that provides reliable measurements of the aortic diameter (Fig. 28.2). US findings correlate closely with operative measurements of AAA, and interobserver variability of less than 5 mm has been demonstrated in 84% of AP measurements (24). Errors and limitations with US are most often attributed to inexperienced technicians, lack of interpretive skills, or excessive bowel gas.
CT is highly predictive of AAA size. Interobserver variability of less than 5 mm exists in 91% of AP measurements. Of importance is that CT may demonstrate other intraabdominal pathologic processes (24). CT is superior to US in assessing AAA wall integrity, the location and amount of calcification within vessel walls, venous anomalies, retroperitoneal blood, aortic dissection, infection or inflammation, and the proximal and distal extent of the aneurysm (Fig. 28.3). Limitations of CT studies include the need for nephrotoxic iodinated contrast administration, radiation exposure, and cost. Spiral or helical CT, an advance over conventional CT, provides excellent resolution
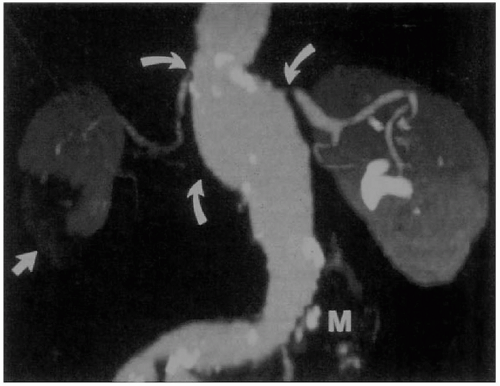
and coronal reconstructions. It has become the study of choice for assessing AAAs before endovascular repair, as well as for identifying postoperative endoleaks in patients treated with endografts (Figs. 28.4 and 28.5).
and coronal reconstructions. It has become the study of choice for assessing AAAs before endovascular repair, as well as for identifying postoperative endoleaks in patients treated with endografts (Figs. 28.4 and 28.5).
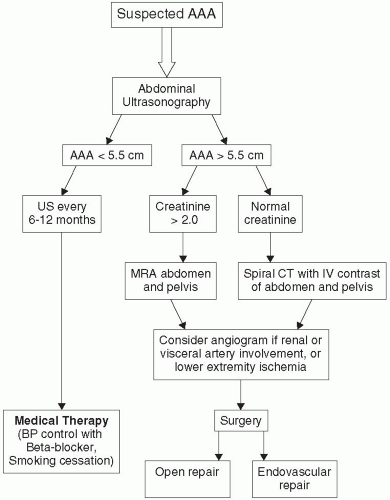 FIGURE 28.1. Algorithm for patient suspected of having an abdominal aortic aneurysm on physical examination. |
Magnetic resonance angiography (MRA) with nonnephrotoxic gadolinium and the use of a breath-holding technique is comparable with CT scanning for AAA measurements (Fig. 28.6). Images are based on T1-weighted images rather than blood flow, which means that slow flow in AAAs does not adversely affect the image. An earlier reported Michigan experience with 43 AAAs revealed that MRA correctly identified maximal AAA diameter and had 94% and 98% sensitivity and specificity, respectively, for identifying significant stenoses of the splanchnic, renal, or iliac arteries (25). MRA limitations include the inability to scan patients who have pacemakers, defibrillators, or claustrophobia, and images obscured by artifacts caused by metallic objects, including certain vascular stents. Another disadvantage of MRA is its inability to image calcified plaque, a finding important in endovascular interventions.
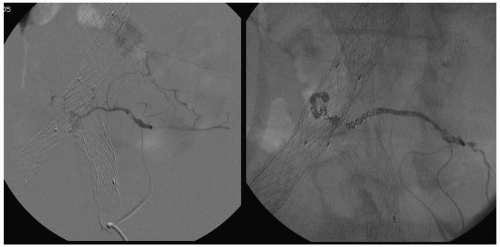
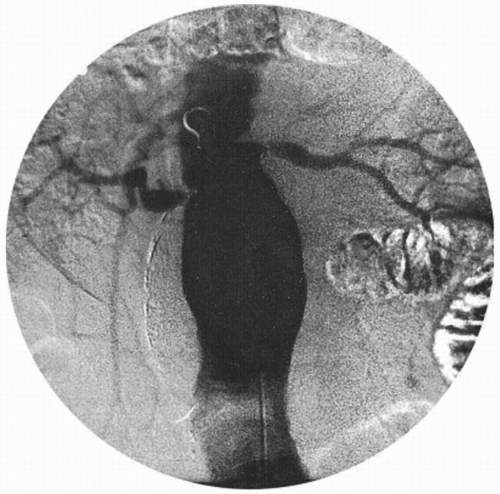
Conventional contrast arteriography and digital subtraction angiography are usually obtained when the AAA is suspected to involve the renal or splanchnic vessels or in patients suspected of having moderate or severe lower extremity ischemia (Fig. 28.7).
These studies identify the cephalad extent of the AAA, the number and location of renal arteries, the state of the splanchnic arteries, and the status of the iliac arteries, as well as the presence of occlusive disease in the lower-extremity arteries. Complications of angiography include bleeding or arterial occlusion at the catheterization site, atheroembolism, and impairment of renal function as a result of iodine contrast nephrotoxicity.
These studies identify the cephalad extent of the AAA, the number and location of renal arteries, the state of the splanchnic arteries, and the status of the iliac arteries, as well as the presence of occlusive disease in the lower-extremity arteries. Complications of angiography include bleeding or arterial occlusion at the catheterization site, atheroembolism, and impairment of renal function as a result of iodine contrast nephrotoxicity.
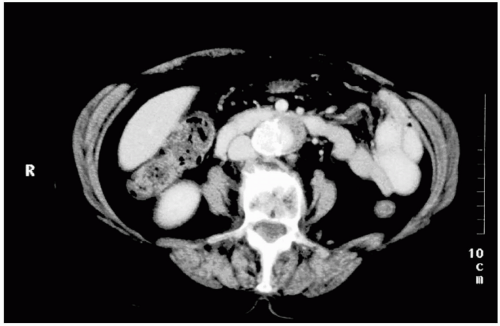 FIGURE 28.3. Computed tomographic scan of the abdomen, demonstrating a mycotic abdominal aortic aneurysm. |
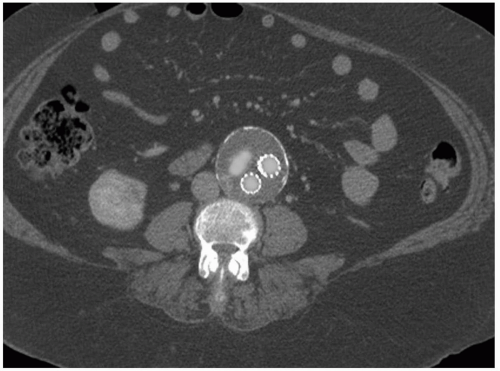 FIGURE 28.4. Computed tomographic scan demonstrating the presence of an endoleak after endovascular repair that resolved after a secondary intervention. |
DIFFERENTIAL DIAGNOSIS
The patient with the unsuspected AAA may be first seen with presumed exacerbation of chronic back pain or with new onset of abdominal, flank, or back pain, radiating to the groin, secondary to acute AAA expansion or rupture. Without imaging studies or a high index of suspicion, this pain may be confused with that of diverticulitis, renal colic, irritable bowel syndrome, inflammatory bowel disease, ovarian torsion, or appendicitis.
SCREENING HIGH-RISK POPULATIONS
The United States Preventive Service Task Force (USPSTF) recently addressed the issue of AAA screening as a measure to diagnose AAAs and prevent the morbidity and mortality associated with rupture. The USPSTF recommends a one-time United States screening for AAA in men aged 65 to 75 years who have ever smoked (current or past use of more than 100 cigarettes). The task force made no recommendation for or against U.S. screening for AAA in men aged 65 to 75 who have never smoked and recommend against routine screening for AAAs in women, as the prevalence of AAA in women is small (26).
COMPLICATIONS
Continued expansion until rupture occurs is the most serious complication of an AAA. It is important to recognize factors contributing to AAA rupture. In accordance with the law of Laplace, a geometric increase in aortic wall pressure occurs with linear increases in AAA size. Thus an increase in aortic diameter from 2 to 4 cm induces, not a twofold, but a fourfold increase in the pressure per square centimeter on the aortic wall. Rupture is directly proportional to aortic wall pressure. It is also known that aortic elastic tissue loses its integrity with age, and acquired or genetic factors that hasten this process add to the risk of accelerated AAA expansion. Aneurysm expansion greater than 4 mm over a 12-month period suggests that the AAA is unstable and is an indication for early intervention.
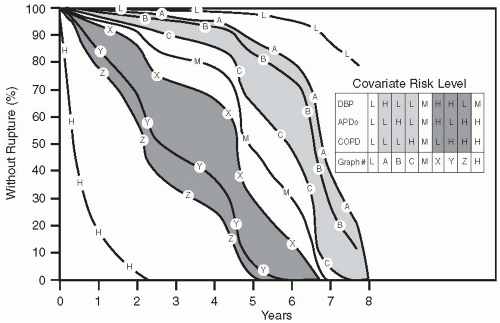
An intact asymptomatic AAA with a diameter of 5 cm is generally recognized to carry a risk of rupture of 20% to 30% over a 2- to 3-year period. The risk of rupture for smaller aneurysms, 3 to 5 cm in size, is less well defined. However, greater AP diameter, chronic obstructive pulmonary disease (COPD), and diastolic hypertension all independently increase the chance of AAA rupture (27). These factors have been assigned low-, medium-, and high-risk (L, M, H) values as follows: for AP diameter, L is 3 cm, M is 4 cm, and H is 5 cm; for COPD, L is none, M is more than 50% predicted forced expiratory volume in 1 second (FEV1), H is less than 50% predicted FEV1; and for diastolic blood pressure, L is 75 mm Hg, M is 90 mm Hg, and H is 105 mm Hg (27). Thus the presence of a 5-cm AAA in a patient with severe diastolic hypertension and COPD is a cause for concern, carrying a
predicted rupture rate of nearly 80% in a year, in comparison with a 3-cm AAA in a normotensive patient without COPD, which carries a rupture rate of only a few percentage points over a 5-year period (Fig. 28.8).
predicted rupture rate of nearly 80% in a year, in comparison with a 3-cm AAA in a normotensive patient without COPD, which carries a rupture rate of only a few percentage points over a 5-year period (Fig. 28.8).
The risk of death after AAA rupture depends on how quickly an emergency operation can be performed. Unfortunately, nearly 60% of patients with ruptured AAAs die before reaching a hospital, and only 50% of the remainder survive an emergency operation. Thus AAA rupture carries an 80% rate of mortality (28,29,30,31).
SURGICAL THERAPY
Elective operative intervention by open surgical repair or endovascular graft placement lessens the likelihood of death from AAA rupture. Conventional open operative procedures in elective circumstances using the National Inpatient Sample database carried a 4.3% mortality rate in 2000 (29). Women fared worse than did men after aneurismectomy for both intact and ruptured aneurysms. Elective mortality over an 11-year period in women was 10.7%, in comparison with 6.8% in men, in this group. The explanation for this is not evident, but suggests both a biologic element and a practice bias, which places women at a disadvantage for the operative treatment of AAA.
Conventional Surgical Treatment of Intact Abdominal Aortic Aneurysm
An expeditious operation is important for an open repair of an AAA (32). An aortic operation longer than 5 hours is independently associated with an increase in risk for mortality and significant cardiopulmonary complications (odds ratio, 5.11; 95% confidence interval, 1.69 to 15.52; p < 0.004). Other factors associated with poor surgical outcome include operative hypothermia, excessive blood loss, and the need for supraceliac aortic cross-clamp. Specific comments about operative technique warrant mention.
Surgical approaches are individualized for each patient, with transperitoneal or retroperitoneal aortic exposure based both on the surgeon’s preference and on the
aortic disease. The transperitoneal approach is preferred when a need exists to revascularize the right kidney or when the aneurysmal disease extends into the right iliac artery. Certain anatomic and clinical circumstances exist in which a retroperitoneal approach may be preferable (33). Relative indications for the retroperitoneal approach include obesity and a history of multiple prior laparotomies, which creates hostile adhesions in the abdomen (33). Although the retroperitoneal approach does not significantly decrease mortality or major cardiopulmonary morbidity, it does expedite the return of postoperative bowel function (34,35).
aortic disease. The transperitoneal approach is preferred when a need exists to revascularize the right kidney or when the aneurysmal disease extends into the right iliac artery. Certain anatomic and clinical circumstances exist in which a retroperitoneal approach may be preferable (33). Relative indications for the retroperitoneal approach include obesity and a history of multiple prior laparotomies, which creates hostile adhesions in the abdomen (33). Although the retroperitoneal approach does not significantly decrease mortality or major cardiopulmonary morbidity, it does expedite the return of postoperative bowel function (34,35).
In the past, thrombotic complications of clamping the aorta and renal failure have been important problems. To address these issues, the patients receive systemic anticoagulants with intravenous heparin before aortic clamping. Diuresis is established, usually with mannitol administration or, in azotemic patients, loop diuretics. Both anticoagulation and a diuresis are established before the aorta is occluded. The aneurysm is then incised, and any intraluminal thrombus is removed before the aortic graft is sewn in place. The prosthetic grafts currently used are either woven or knitted Dacron or extruded Teflon. After the graft is in place and blood flow is restored to the lower body, it is important that the graft be covered with the aneurysm shell or other retroperitoneal tissue, to prevent contact with the intestines. Such contact may lead to later graft-enteric erosion, which is considered a life-threatening complication necessitating graft removal. All patients with aortic grafts should receive antibiotics for invasive procedures, including dental procedures performed at a later date, similar to prophylaxis for bacterial endocarditis in patients with prosthetic cardiac valves.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree