N = 100 (values are also %)
Age median (range)
30 years (1–89)
Male
70
Hypotensive on admission (<90 mmHg)
34
Cardiac arrest in route, ED or OR
14
Associated injuries
Traumatic brain injury
11
Rib fractures
10
Pneumothorax/hemothorax
13
Abdominal wall ecchymosis
34
Abdominal wall degloving
9
Solid organ injury
25
Mesenteric hematoma/laceration
26
Small bowel injury
38
Colon injury
22
Spine fracture
32
Pelvic fracture
16
IVC injury
8
Retroperitoneal hematoma
50
Lower extremity ischemia
17
Location on aorta
Zone I: superior to SMA
8
Zone II: SMA to RA
6
Zone III: infrarenal
86
16.2 Anatomy/Physiology
Abdominal aortic mechanism of injury relates to the biomechanical direct and indirect forces incurred while it is tethered between the spinal column, the peritoneum, and abdominal viscera [5, 6]. The most frequent force causing an abdominal aorta injury is compressive, associated with deceleration [55]. Indirect forces occur when the aortic wall is directly stretched and compressed against a high-pressure column of blood leading to intimal tears, pseudoaneurysm, rupture, or thrombosis [56, 57]. Atherosclerotic changes of the aorta are associated with a weakening of the intima in addition to loss of elasticity and compliance and thus thought to increase the risk of aortic injury [58, 59]. These forces can occur during motor vehicle collisions when the aorta is compressed by the seat belt, thus termed “seat belt aorta” [60]. Other mechanisms include long-distance falls, direct compression of the aorta, and penetrating injuries.
The underlying pathologic lesion of BAAI is the disruption of the intima. Depending on the magnitude of the traumatic forces, BAAI presents as a spectrum of disease ranging from a minimal aortic injury (MAI) to free rupture of the aorta [61]. The increasing use of CT scanning with angiography to evaluate patients with blunt trauma has resulted in increasing numbers of MAI diagnosed [61]. These traumatic intimal tears can manifest as intimal flaps or dissection which can be uncomplicated or progress to thrombosis or acute arterial insufficiency [21, 22, 29, 56]. Injuries involving the adventitia lead to pseudoaneurysm formation (Fig. 16.1) or even rupture of the aortic wall. Rupture of the aortic wall can also be due to branch vessel avulsion [2].
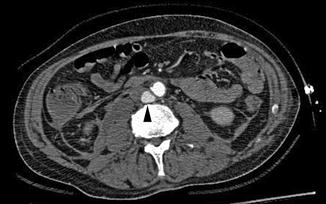

Fig. 16.1
Blunt abdominal aortic pseudoaneurysm (arrowhead) as seen on axial view with CT angiography
The majority of the injuries occur inferior to the renal arteries. Aortic injuries are classified based on presence of aortic contour abnormality and presence of free rupture [5]. This classification is based on experience with blunt thoracic aortic injury [62]. Intimal tears/flaps are not associated with external aortic contour abnormality. A pseudoaneurysm presents as a contained rupture and is clearly associated with an aortic contour abnormality. The most common associated injuries are small bowel injuries (38 %) and thoracolumbar spine injuries (25 %). The most common anatomic findings since 1996 were intimal tears/flaps (41 %) followed by reports of pseudoaneurysms (29 %) [8, 10, 11, 15–47].
16.2.1 Clinical Assessment and Diagnostic Testing
The initial presentation is dependent on presence or absence of free rupture of the abdominal aorta, branch vessel avulsion, or concomitant inferior vena cava injury [2]. Those with rupture typically present with hypotension due to hemorrhagic shock. Emergency room anterolateral thoracotomy is used to provide cross clamping the aorta in the distal thorax. In our recent BAAI series, all patients with rupture were hypotensive, and 75 % had cardiac arrest in the emergency department with 38 % requiring an emergency department thoracotomy [5]. Tamponade is usually associated with a retroperitoneal hematoma which improves survival [2, 3]. Abdominal wall ecchymosis is seen in one third of BAAI cases and should raise the index of suspicion for aortic and associated hollow viscus injuries [5].
16.2.2 Diagnostic Testing
Diagnostic testing is dependent on the initial presentation. In the hypotensive patient, the focused assessment with sonography for trauma (FAST) scan or a diagnostic peritoneal lavage (DPL) is the first step. If significant hemoperitoneum is present, then operative exploration is warranted. In the temodynamically stable patient an abdominal and pelvic CT scan with contrast is done allowing the evaluation of the aortic anatomy and concomitant injuries [63].
16.3 Initial Management
Management of aortic injury varies and risk of mortality rises with the severity of the pathologic lesion. In a recent review of the National Trauma Data Bank of 436 patients with BAAI from 180 centers from 2007 to 2009, 90 % were managed nonoperatively. Of those who underwent operative repair, 69 % underwent endovascular repair, with the remainder undergoing open aortic repair and two extra-anatomic bypasses [64]. In general, cases of BAAI with uncomplicated intimal flaps are managed nonoperatively with anti-impulse (β-blockers) and antiplatelet (aspirin) therapy and close follow-up. The natural history of intimal flaps is a decrease in size and complete resolution [9]. Cases of aortic pseudoaneurysms require operative repair to prevent rupture, but these can be managed on a semi-elective basis during the initial hospitalization. Cases complicated by thrombosis, acute arterial insufficiency, and free rupture require an operative or endovascular repair.
The timing of repair also depends on the patient’s associated injuries. Patients with pulse and neurologic deficits, an expanding hematoma and concomitant intra-abdominal injuries, require emergent exploration and immediate repair. Otherwise, treatment can be individualized in the hemodynamically normal trauma patient.
16.4 Open Repair
Initial management is determined by the hemodynamic state of the patient. Hypotensive patients are managed with an exploratory laparotomy. A midline laparotomy incision allows exposure of all zones of the abdomen. Initial inspection identifies areas of ongoing hemorrhage, contained hematoma, or evidence of bowel ischemia [3]. Operative management of aortic injury is guided by its associated Zone 1 hematoma which may be supramesocolic or inframesocolic. Common pitfalls and pearls regarding management of the patient with an aortic injury are discussed below.
16.4.1 Exposure and Control of the Aorta
Exposure is guided by the location of the retroperitoneal hematoma. In the case of a supramesocolic hematoma, proximal control of the aorta can be obtained at the diaphragm. The supraceliac aorta is exposed by opening the gastrohepatic ligament, lateral retraction of the left lobe of the liver, and caudal retraction of the stomach. The esophagus is mobilized laterally, its location facilitated by the presence of a nasogastric or orogastric tube. This allows identification of the abdominal aorta at the diaphragmatic hiatus. Control is achieved manually with compression or by vascular clamp placement (Fig. 16.2). Full exposure of the supraceliac aorta and its branches is achieved with left medial visceral rotation (the Mattox maneuver). For an inframesocolic hematoma, the exposure is obtained in a transperitoneal fashion similar to that for an infrarenal aneurysm. This is achieved with caudal retraction of the transverse colon, retraction of the small intestine to the right, and cephalad mobilization of the third and fourth portions of the duodenum. The proximal extent of this exposure is the left renal vein which can be divided if necessary between clamps for more cephalad access to the aorta. Proximal control of the aorta is obtained immediately below the renal arteries or at the diaphragm [65].
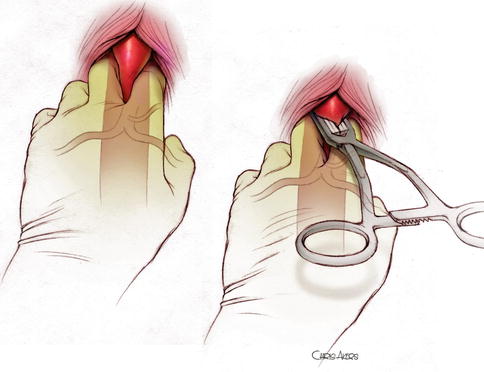

Fig. 16.2
Manual clamping of the supraceliac aorta, followed by placement of an atraumatic vascular clamp over the fingers
16.4.2 Repair of the Aorta
Repair of the aorta is determined by extent of injury and on presence of gross contamination from hollow viscus injury. In cases of aortic tears, multiple injuries can be connected and closed in linear fashion using polypropylene suture, Dacron patch aortoplasty, or Dacron graft interposition depending on the size of the tear [65]. In cases of intimal flaps complicated by thrombosis and acute arterial insufficiency, repair is accomplished with thrombectomy and tacking sutures of the intimal flap or Dacron graft interposition if the damage to the intima is substantial (Fig. 16.3). Pseudoaneurysms usually require excision with primary end-to-end anastomosis or interposition grafting.
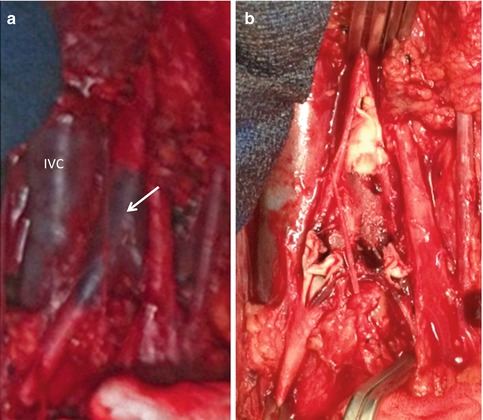

Fig. 16.3
Blunt abdominal aortic injury associated with thrombosis of the infrarenal aorta (arrow) and bilateral proximal iliac arteries (a). Complete intimal disruption seen (b)
Gross contamination from hollow viscus injury can jeopardize aortic grafts due to risk of infection. Thus ligation of the injured aorta with extra-anatomic bypass, such as axillobifemoral bypass, may be required. In cases in which damage control laparotomy is needed, shunts such as chest tubes, endotracheal tubes, or carotid shunts can be used to establish temporary control, in a damage control fashion until hypothermia, coagulopathy, and acidosis are corrected.
16.5 Endovascular Management of Aortic Injuries
Reports of endovascular stent placement in cases of aortic injuries have recently increased in frequency because these techniques are less invasive and an attractive alternative for patients with both isolated aortic injuries and multisystem injuries in a manner similar to that of blunt thoracic aortic injury (Fig. 16.4). The use of stent grafts is not widely adopted, and most of the literature is limited to case reports and small case series where endovascular stents, aortic extension cuffs, and limb extensions have all been used in cases of blunt and penetrating trauma [23, 24, 29, 31, 34, 35, 44, 66–69]. Indications include cases with associated gross contamination from hollow viscus injury that can jeopardize aortic grafts due to risk of infection or management of injuries difficult to expose by conventional means. Endovascular interventions can be used as a stabilizing measure for critically ill patients and a bridge to open definitive repair. From an endovascular perspective as it relates to trauma, abdominal aorta zones of injury can be classified [5] based on feasibility of endovascular approach as follows: Zone I injuries occur from diaphragmatic hiatus to the superior mesenteric artery (SMA). Zone II injuries include the SMA to renal arteries. Zone III injuries are inferior to the renal arteries to the aortic bifurcation (Fig. 16.5). Zone I and III injuries are amenable to endovascular repair, whereas Zone II lesions are not amenable to endovascular stenting without fenestration for the SMA and renal arteries. The timing of the repair is dependent on the patient’s hemodynamic status and the presence of acute limb ischemia. Endovascular repair can be used for injuries from the diaphragmatic hiatus to the superior mesenteric artery or for injuries below the renal arteries to the aortic bifurcation. Injuries that involve the aorta in the vicinity of the SMA and renal arteries are not easy to manage by an endovascular technique as this would require graft fenestration for the SMA and renal arteries
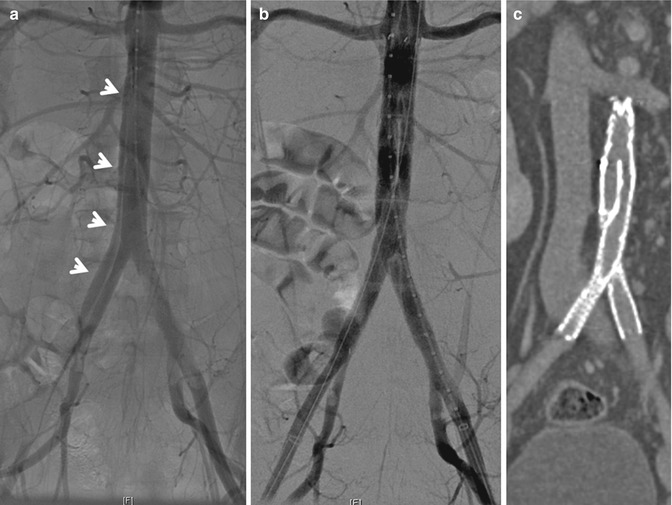
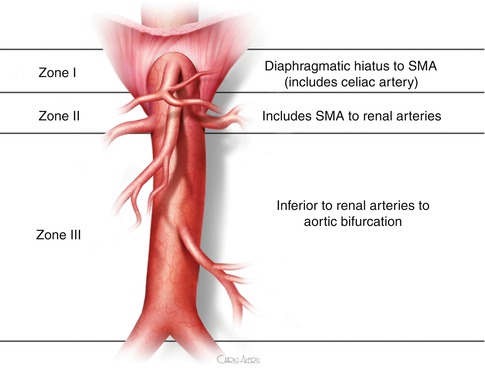

Fig. 16.4
Blunt abdominal aortic dissection. (a) An intraoperative arteriogram showing the dissection flap (arrows) in the infrarenal aorta and extending to the right common iliac artery. (b) Arteriogram post endovascular stent graft placement. (c) Coronal view of CT angiography 6 weeks post repair

Fig. 16.5
Zone I injuries occur from diaphragmatic hiatus to the superior mesenteric artery (SMA). Zone II injuries include the SMA to Renal arteries. Zone III injuries are inferior to the renal arteries to the aortic bifurcation
16.5.1 Intra-Aortic Occlusion Balloon (IAOB)
The most common cause of death from aortic trauma remains hemorrhagic shock compounded by ongoing coagulopathy; thus early proximal control of the aorta is a key maneuver [2, 5, 6, 10]. The use of transfemoral IAOB to obtain proximal control at the level of the diaphragm prior to entering the abdomen may have a role in early control of hemorrhage from the injured aorta [70]. Placement of the IAOB can be done expeditiously and would not delay the laparotomy as demonstrated in proximal control of the aorta in cases of ruptured abdominal aortic aneurysms [71]. Current studies are evaluating the use of this modality in animal models [72, 73].
16.6 Pearls, Complications, and Pitfalls
Patient preparation includes a warmed room to minimize patient hypothermia.
Placement of the patient on a radiolucent table allows the option for endovascular intervention. Prior to incision, a nasogastric or orogastric tube is placed to decompress the stomach and facilitate identification of the esophagus during supraceliac aortic clamping. A Foley catheter is inserted to decompress the bladder and avoid injury during laparotomy as well as for urine output monitoring.
Concomitant limb ischemia may be present due to intimal flaps complicated by thrombosis and acute arterial insufficiency; thus it is imperative to assess the peripheral vasculature for involvement of limb ischemia prior to the start of the case and at the conclusion of the procedure.
The patient is prepped from sternal notch to upper anterior thigh. This allows access to the anterior thorax should a thoracic injury be identified or if vascular control via the thorax is needed. Distally preparing the upper anterior thighs allows for saphenous vein harvest should vascular reconstruction be deemed necessary.
Broad spectrum antibiotic is administered prior to incision.
16.7 Outcomes
Injury to the abdominal aorta is highly lethal. Among those who survive the transport to the hospital mortality rates range from 32 to 78 % with hemorrhagic shock being the most common cause of associated mortality [2, 4–6, 10]. Outcomes in cases requiring a resuscitative thoracotomy remain poor [74, 75]. In BAAI, mortality varies by the type of aortic injury. When minimal aortic injuries, dissections of the aorta, and pseudoaneurysms are included, aggregate mortality is 11 % [5]. In cases treated by endovascular repair, the long-term durability of aortic endografts for abdominal aortic trauma is not well described. Clearly long-term follow-up will be required in these cases.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


