Diabetes mellitus (DM) is a risk factor for incident heart failure (HF) in older adults. However, the extent to which this association is independent of other risk factors remains unclear. Of 5,464 community-dwelling adults ≥65 years old in the Cardiovascular Health Study without baseline HF, 862 had DM (fasting plasma glucose levels ≥126 mg/dl or treatment with insulin or oral hypoglycemic agents). Propensity scores for DM were estimated for each of the 5,464 participants and were used to assemble a cohort of 717 pairs of participants with and without DM who were balanced in 65 baseline characteristics. Incident HF occurred in 31% and 26% of matched participants with and without DM, respectively, during >13 years of follow-up (hazard ratio 1.45 for DM vs no DM, 95% confidence interval [CI] 1.14 to 1.86, p = 0.003). Of the 5,464 participants before matching unadjusted and multivariable-adjusted hazard ratios for incident HF associated with DM were 2.22 (95% CI 1.94 to 2.55, p <0.001) and 1.52 (95% CI 1.30 to 1.78, p <0.001), respectively. All-cause mortality occurred in 57% and 47% of matched participants with and without DM, respectively (hazard ratio 1.35, 95% CI 1.13 to 1.61, p = 0.001). Of matched participants DM-associated hazard ratios for incident peripheral arterial disease, incident acute myocardial infarction, and incident stroke were 2.50 (95% CI 1.45 to 4.32, p = 0.001), 1.37 (95% CI 0.97 to 1.93, p = 0.072), and 1.11 (95% CI 0.81 to 1.51, p = 0.527), respectively. In conclusion, the association of DM with incident HF and all-cause mortality in community-dwelling older adults without HF is independent of major baseline cardiovascular risk factors.
Diabetes mellitus (DM) is a major risk factor for incident heart failure (HF). However, DM is also associated with many traditional cardiovascular risk factors. The extent to which the association of DM with incident HF is independent of other cardiovascular risk factors remains unclear. Although traditional multivariable risk adjustment models can account for baseline differences in the distribution of such risk factors, they cannot guarantee that they would be balanced. Propensity-score matching, in contrast, can be used for outcome-blinded assembly of study cohorts in which exposed and unexposed groups are balanced in all measured baseline characteristics. Therefore, we conducted a propensity-matched study of the association of DM with incident HF, mortality, and incident cardiovascular events.
Methods
The Cardiovascular Health Study (CHS) is a National Heart, Lung, and Blood Institute–funded prospective study designed to assess traditional and nontraditional cardiovascular risk factors in community-dwelling older adults. The CHS recruited 5,888 Medicare-eligible community-dwelling adults ≥65 years of age from 4 United States communities in 2 phases. A mostly white initial cohort of 5,201 participants (1989 through 1990) was later supplemented by 687 African-Americans from 3 of those 4 communities (1992 through 1993). We used a de-identified public-use copy of the CHS dataset obtained from the National Heart, Lung, and Blood Institute, which contained information on 5,795 participants who consented to be included in that dataset. After excluding 63 participants without data on DM status and 268 participants with prevalent HF at baseline, the final sample for the present analysis was 5,464 participants.
Baseline DM was defined by a fasting plasma glucose level >126 mg/dl or treatment with insulin or hypoglycemic drugs and 16% of CHS participants (862 of 5,464) had DM. Data on sociodemographic, clinical, subclinical, and laboratory variables including serum insulin, triglyceride, interleukin-6, and C-reactive protein levels were measured at baseline. If the value of a continuous variable was found to be missing, then predicted values based on age, gender, and race were imputed. The primary outcome for this study was incident HF, which was centrally adjudicated by the CHS events committee. Data on self-reports of physician diagnosis of HF were obtained during semiannual visits, which was then verified by review of medical records. Secondary outcomes included all-cause and cause-specific mortalities, acute myocardial infarction (AMI), stroke, and peripheral arterial disease.
Propensity scores, or the conditional probability of having DM, were estimated for each of the 5,464 participants using a nonparsimonious multivariable logistic regression model in which DM was the dependent variable and the 65 baseline characteristics were covariates. We then used propensity scores to match 717 participants (83% of 862) with DM to 717 of those without DM who had similar propensity scores. Absolute standardized differences before and after matching for all 65 covariates were estimated and presented as a Love plot ( Figure 1 ). An absolute standardized difference <10% indicates inconsequential imbalances, whereas 0% indicates no between-group imbalances on that covariate.
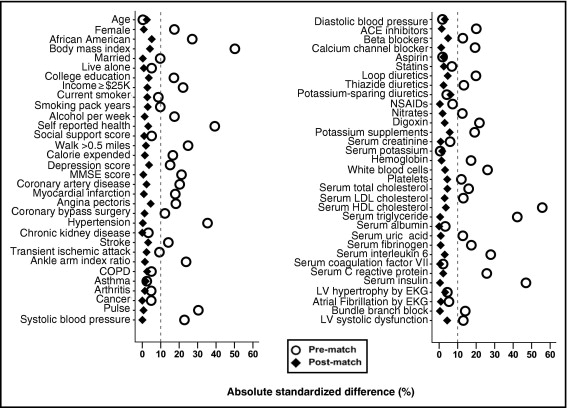
For between-group comparisons for data before and after matching we used Pearson chi-square tests, Wilcoxon rank-sum tests, McNemar tests, and paired-sample t tests, as appropriate. Kaplan–Meier and matched Cox proportional hazard analyses were used to estimate associations between DM and outcomes. Formal sensitivity analyses were conducted to determine the impact of a potential hidden confounder on the association between DM and incident HF in the matched cohort. Subgroup analyses were performed to determine the homogeneity of this association. Two-tailed statistical tests with 95% confidence intervals (CIs) were employed with a p value <0.05 considered statistically significant. All data analysis was completed using SPSS 15 for Windows (SPSS, Inc., Chicago, Illinois).
Results
Our matched cohort had a mean age ± SD of 73 ± 6 years, 51% were women, and 21% were African-American ( Table 1 ). Before matching, participants with DM were more likely to have a history of coronary artery disease, hypertension, and stroke and higher mean serum insulin, triglyceride, interleukin-6, and C-reactive protein levels. These and other imbalances were balanced in the matched cohort ( Figure 1 , Table 1 ).
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| DM | p Value | DM | p Value | |||
| No | Yes | No | Yes | |||
| (n = 4,602) | (n = 862) | (n = 717) | (n = 717) | |||
| Age (years) | 73 ± 6 | 73 ± 5 | 0.936 | 73 ± 5 | 73 ± 6 | 0.646 |
| Women | 2,714 (59%) | 434 (50%) | <0.001 | 364 (51%) | 367 (51%) | 0.916 |
| African-American | 610 (13%) | 204 (24%) | <0.001 | 144 (20%) | 159 (22%) | 0.361 |
| Body mass index (kg/m 2 ) | 26 ± 4 | 28 ± 4 | <0.001 | 28 ± 4 | 28 ± 4 | 0.409 |
| Married | 3,101 (67%) | 541 (63%) | 0.008 | 463 (65%) | 462 (64%) | 1.000 |
| Current smoker | 580 (13%) | 85 (10%) | 0.024 | 79 (11%) | 73 (10%) | 0.666 |
| Smoking (pack-years) | 17 ± 26 | 20 ± 30 | 0.006 | 19 ± 28 | 19 ± 28 | 0.581 |
| Alcohol intake (units/week) | 3 ± 7 | 2 ± 5 | <0.001 | 2 ± 6 | 2 ± 5 | 0.800 |
| General health, fair to poor | 948 (21%) | 329 (38%) | <0.001 | 258 (36%) | 247 (34%) | 0.541 |
| Medical history | ||||||
| Coronary artery disease | 743 (16%) | 209 (24%) | <0.001 | 172 (24%) | 165 (23%) | 0.709 |
| Acute myocardial infarction | 332 (7%) | 108 (13%) | <0.001 | 87 (12%) | 85 (12%) | 0.935 |
| Angina pectoris | 617 (13%) | 174 (20%) | <0.001 | 148 (21%) | 135 (19%) | 0.426 |
| Coronary artery bypass surgery | 154 (3%) | 51 (6%) | <0.001 | 40 (6%) | 38 (5%) | 0.905 |
| Hypertension | 2,556 (56%) | 623 (72%) | <0.001 | 505 (70%) | 506 (71%) | 1.000 |
| Chronic kidney disease | 952 (21%) | 190 (22%) | 0.369 | 152 (21%) | 152 (21%) | 1.000 |
| Stroke | 154 (3%) | 55 (6%) | <0.001 | 34 (5%) | 39 (5%) | 0.620 |
| Transient ischemic attack | 107 (2%) | 34 (4%) | 0.006 | 27 (4%) | 24 (3%) | 0.775 |
| Peripheral arterial disease | 511 (11%) | 169 (20%) | <0.001 | 130 (18%) | 134 (19%) | 0.832 |
| Chronic obstructive pulmonary disease | 581 (13%) | 95 (11%) | 0.189 | 86 (12%) | 80 (11%) | 0.685 |
| Cancer | 669 (15%) | 111 (13%) | 0.201 | 93 (12%) | 93 (13%) | 1.000 |
| Clinical examination | ||||||
| Pulse rate (beats/min) | 67 ± 11 | 71 ± 12 | <0.001 | 70 ± 11 | 70 ± 12 | 0.895 |
| Systolic blood pressure (mm Hg) | 136 ± 21 | 141 ± 21 | <0.001 | 140 ± 22 | 140 ± 21 | 0.990 |
| Diastolic blood pressure (mm Hg) | 71 ± 11 | 71 ± 12 | 0.631 | 71 ± 11 | 71 ± 12 | 0.558 |
| Medications | ||||||
| Angiotensin-converting enzyme inhibitor | 254 (6%) | 95 (11%) | <0.001 | 77 (11%) | 74 (10%) | 0.857 |
| β Blocker | 553 (12%) | 142 (17%) | <0.001 | 130 (18%) | 117 (16%) | 0.411 |
| Calcium channel blocker | 527 (12%) | 158 (18%) | <0.001 | 129 (18%) | 126 (18%) | 0.891 |
| Statin | 94 (2%) | 27 (3%) | 0.046 | 22 (3%) | 19 (3%) | 0.755 |
| Loop diuretic | 183 (4%) | 76 (9%) | <0.001 | 62 (9%) | 53 (7%) | 0.444 |
| Thiazide diuretic | 489 (11%) | 130 (15%) | <0.001 | 109 (15%) | 103 (14%) | 0.701 |
| Nitrate | 323 (7%) | 91 (11%) | <0.001 | 69 (10%) | 73 (10%) | 0.789 |
| Digoxin | 259 (6%) | 101 (12%) | <0.001 | 59 (8%) | 65 (9%) | 0.631 |
| Laboratory values | ||||||
| Creatinine (mg/dl) | 0.95 ± 0.32 | 1.00 ± 0.60 | 0.001 | 0.99 ± 0.34 | 0.99 ± 0.64 | 0.877 |
| Potassium (mEq/L) | 4.16 ± 0.37 | 4.16 ± 0.41 | 0.880 | 4.17 ± 0.40 | 4.16 ± 0.40 | 0.764 |
| Cholesterol (mg/dl) | 213 ± 38 | 206 ± 42 | <0.001 | 205 ± 39 | 207 ± 42 | 0.398 |
| Low-density lipoprotein (mg/dl) | 131 ± 35 | 126 ± 38 | <0.001 | 126 ± 34 | 127 ± 37 | 0.574 |
| High-density lipoprotein (mg/dl) | 56 ± 16 | 48 ± 13 | <0.001 | 48 ± 12 | 48 ± 13 | 0.468 |
| Triglyceride (mg/dl) | 133 ± 67 | 172 ± 111 | <0.001 | 163 ± 93 | 163 ± 93 | 0.915 |
| Uric acid (mg/dl) | 5.6 ± 1.5 | 5.8 ± 1.5 | 0.001 | 5.8 ± 1.5 | 5.8 ± 1.5 | 0.868 |
| C-reactive protein (mg/L) | 4.2 ± 7.0 | 6.8 ± 12.3 | <0.001 | 6.1 ± 10.7 | 5.9 ± 8.2 | 0.671 |
| Insulin (IU/ml) | 14 ± 8 | 32 ± 56 | <0.001 | 20 ± 15 | 20 ± 13 | 0.879 |
| Interleukin-6 (pg/ml) | 2.1 ± 1.8 | 2.6 ± 1.7 | <0.001 | 2.4 ± 1.8 | 2.5 ± 1.6 | 0.560 |
| Hemoglobin (g/dl) | 14 ± 1 | 14 ± 1 | <0.001 | 14 ± 1 | 14 ± 1 | 0.809 |
| White blood cell count (10 3 /μl) | 6.2 ± 2.0 | 6.8 ± 2.7 | <0.001 | 7 ± 3 | 7 ± 2 | 0.547 |
| Platelets (10 3 /μl) | 252 ± 75 | 243 ± 75 | 0.001 | 247 ± 73 | 244 ± 75 | 0.410 |
| Electrocardiographic findings | ||||||
| Left ventricular hypertrophy | 192 (4%) | 44 (5%) | 0.217 | 38 (5%) | 33 (5%) | 0.625 |
| Atrial fibrillation | 91 (2%) | 24 (3%) | 0.130 | 17 (2%) | 16 (2%) | 1.000 |
| Bundle branch block | 357 (8%) | 103 (12%) | <0.001 | 84 (12%) | 83 (12%) | 1.000 |
| Left ventricular systolic dysfunction | 318 (7%) | 93 (11%) | <0.001 | 62 (9%) | 71 (10%) | 0.478 |
Incident HF occurred in 31% and 26% of matched participants with and without DM, respectively, during >13 years of follow-up (hazard ratio 1.45, 95% CI 1.14 to 1.86, p = 0.003; ( Figure 2 , Table 2 ). A hidden binary covariate that is a near-perfect predictor of incident HF would need to increase the odds of DM by 23% to explain away this association. This association was homogenous across various subgroups of matched participants except that it was stronger in those without hypertension than in those with hypertension ( Figure 3 ). Associations of DM with incident HF before matching are listed in Table 2 .
| Percentage (events/total) | Absolute Risk Difference ⁎ (%) | HR † (95% CI) | p Value | ||
|---|---|---|---|---|---|
| No DM | DM | ||||
| Unadjusted | 19% (862/4,602) | 32% (272/862) | +13% | 2.22 (1.94–2.55) | <0.001 |
| Multivariable adjusted | — | — | — | 1.52 (1.30–1.78) | <0.001 |
| Propensity matched | 26% (183/717) | 31% (220/717) | +5% | 1.45 (1.14–1.86) | 0.003 |
⁎ Absolute risk differences were calculated by subtracting percent events in the no-diabetes group from that in the diabetes group.
All-cause mortality in the postmatch cohort occurred in 57% and 47% of participants with and without DM, respectively (hazard ratio 1.35, 95% CI 1.13 to 1.61, p = 0.001; Figure 2 , Table 3 ). Associations of DM with cardiovascular and noncardiovascular mortalities are presented in Table 3 . Associations of DM with other incident cardiovascular outcomes are presented in Table 4 . Of those who developed incident HF only 25 patients (8%) had incident AMI before HF, which occurred in 1% (6 of 630) and 3% (19 of 632) of those with and without DM, respectively (p = 0.009).
| Percentage (events/total) | Absolute Risk Difference ⁎ (%) | HR † (95% CI) | p Value | ||
|---|---|---|---|---|---|
| No DM | DM | ||||
| All-cause mortality | |||||
| Unadjusted | 41% (1,895/4,602) | 59% (509/862) | +18% | 1.82 (1.65–2.01) | <0.001 |
| Multivariable adjusted | — | — | — | 1.44 (1.29–1.62) | <0.001 |
| Propensity matched | 47% (334/717) | 57% (408/717) | +10% | 1.35 (1.13–1.61) | 0.001 |
| Cardiovascular mortality | |||||
| Unadjusted | 16% (724/4,602) | 29% (248/862) | +13% | 2.31 (2.00–2.67) | <0.001 |
| Multivariable adjusted | — | — | — | 1.65 (1.40–1.95) | <0.001 |
| Propensity matched | 20% (142/717) | 27% (192/717) | +7% | 1.53 (1.23–1.91) | <0.001 |
| Noncardiovascular mortality | |||||
| Unadjusted | 25% (1,165/4,602) | 30% (260/862) | +5% | 1.52 (1.33–1.74) | <0.001 |
| Multivariable adjusted | — | — | — | 1.32 (1.13–1.53) | <0.001 |
| Propensity matched | 27% (190/717) | 30% (215/717) | +3% | 1.30 (1.07–1.58) | 0.008 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


