The aim of this study was to investigate the value of a new Revised Cardiac Risk Index (RCRI) that includes consideration of QRS fragmentation (fQRS) as a predictor of cardiac events in patients undergoing noncardiac vascular surgery. Four hundred sixty-seven consecutive patients admitted for noncardiac vascular surgery were studied. Patients were allocated to RCRI 0, 1, 2, or ≥3 groups according to the sum of diabetes, renal insufficiency, and histories of ischemic heart disease, congestive heart failure, and cerebrovascular disease. They were then reallocated to fragmented RCRI (fRCRI) 0, 1, 2, or ≥3 groups after including a score of 1 or 0 corresponding to the presence or absence of fQRS. A major adverse cardiac event (MACE) was defined as a composite of death, myocardial infarction, congestive heart failure, and percutaneous coronary intervention before noncardiac vascular surgery. During index hospitalization, MACE developed in 38 patients (8.1%). fQRS was present in 169 (36.2%), and it was significantly greater in patients with MACE than in those without MACE (63.2% vs 34.3%, p <0.001). The proportions of RCRI 0, 1, 2, and ≥3 were 46.9% (n = 219), 35.3% (n = 165), 12.4% (n = 58), and 5.4% (n = 25), respectively. When fRCRI data were included, 28 patients (48.3%) in RCRI 2 were reclassified as fRCRI ≥3. By multivariate logistic regression analysis, fRCRI (odds ratio 1.529, 95% confidence interval 1.035 to 2.258, p = 0.033) and a left ventricular ejection fraction <50% independently predicted in-hospital MACE. In conclusion, fRCRI is an independent predictor of in-hospital MACE in patients undergoing noncardiac vascular surgery.
Perioperative cardiac complications such as acute coronary syndrome, pulmonary edema, and serious arrhythmia are major causes of mortality and morbidity in patients with multiple cardiac risk factors undergoing surgery. The Revised Cardiac Risk Index (RCRI) is used for stratifying patient risk before surgery, and the American College of Cardiology/American Heart Association Guidelines for perioperative risk assessment recommend preoperative noninvasive stress imaging in patients at high cardiac risk based on RCRI score before vascular surgery. However, previous studies have demonstrated that the prognostic role of RCRI in terms of predicting the development of perioperative cardiac events is limited, which suggests the need for an additional marker with greater prognostic power. Fragmented QRS complex (fQRS) is a novel marker of cardiovascular disease and has been shown to predict cardiac events, including death and heart-failure progression after acute coronary syndrome and arrhythmia in several disease entities. However, nothing is known of the ability of fQRS to predict cardiac events in high-risk patients undergoing noncardiac vascular surgery. Accordingly, in the present study, we investigated associations between fQRS and a newly reclassified RCRI that includes consideration of fQRS (fRCRI) and perioperative cardiac events in patients undergoing noncardiac vascular surgery.
Methods
Five hundred forty-three consecutive patients who underwent myocardial perfusion single-photon emission computed tomography (SPECT) for noncardiac vascular surgery at Kyungpook National University Hospital (Daegu, Korea) from January 2006 to March 2012 were retrospectively enrolled. The association between fRCRI and myocardial ischemia or scarring as determined by myocardial SPECT has been previously reported. Demographic and clinical characteristics, including age, gender, body mass index, cardiovascular risk factors (hypertension, diabetes, hyperlipidemia, current smoking), and co-morbidities (previous ischemic heart disease, congestive heart failure, cerebrovascular disease) were identified. Peripheral blood samples for laboratory tests were obtained by direct venipuncture, and renal insufficiency was defined as a serum creatinine level of ≥2 mg/dl. We calculated RCRI scores for each patient by assigning 1 point for the following 5 risk factors: all types of diabetes; renal insufficiency; and histories of ischemic heart disease, congestive heart failure, and cerebrovascular disease. We then classified patients as RCRI 0, 1, 2, or ≥3 based on the number of risk factors present. We analyzed fQRS using 12-lead electrocardiogram (ECG) and assigned 1 point for the presence of fQRS. We then reclassified patients by fRCRI values of 0, 1, 2, or ≥3 by summing the RCRI and the fQRS score. The presence of myocardial ischemia or scarring was determined by myocardial perfusion SPECT. A perioperative major adverse cardiac event (MACE) was defined as a composite of death, myocardial infarction, congestive heart failure, and percutaneous coronary intervention before noncardiac vascular surgery during index hospitalization. The additional value of fRCRI versus RCRI was evaluated. The study was approved by our institutional ethics committee.
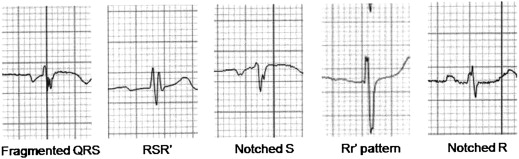
Among 12-lead ECGs (Philips TraceMasterVue ECG management system, Philips 12-lead algorithm, Andover, Massachusetts; filter range, 0.5 to 150 Hz; alternating current filter 60 Hz; 25 mm/s; 10 mm/mV) obtained at rest before surgery, an ECG obtained on the nearest surgical day was analyzed by 2 independent cardiologists. Disagreements were resolved by consensus. fQRS was defined as changes in QRS morphology (<120 ms) with different RSR′ patterns, that is, additional R (R′) waves, notching, S waves, or >1 R′ waves in 2 contiguous leads. In a bundle branch block pattern with a QRS duration of ≥120 ms, fQRS was defined as different RSR′ patterns, that is, >2 R waves (R′), >2 notches in R waves, or S waves in 2 contiguous leads. Incomplete bundle branch blocks were excluded. A pathologic Q wave was defined by the presence of a Q wave deeper than 1/4 the size of the voltage of the subsequent R wave, or >0.04 second in duration.
Data are expressed as means ± SDs for continuous variables and as percentages for categorical variables. All comparisons between baseline variables were assessed using the Student t test for continuous variables or with Pearson chi-square test for categorical variables. The different distributions of RCRI and fRCRI were compared using the paired-samples t test. A multivariate logistic regression model was used to identify independent predictors of perioperative MACE. Incremental factors added to the model at each step were considered significant when differences in log-likelihoods associated with models corresponded to p <0.05. We estimated receiver operating characteristic curves and compared areas under curves (with 95% confidence interval) in corresponding logistic models. For all analyses, a 2-sided p <0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 15.0 for Windows (SPSS Inc., Chicago, Illinois), and MedCalc version 12.3 (MedCalc Software, Ostend, Belgium).
Results
Of the 543 consecutive patients who underwent myocardial perfusion SPECT for preoperative evaluation of noncardiac vascular surgery, 76 patients were excluded from the analysis because patients or the physicians refused surgery. Finally, 467 patients (mean age 69.4 ± 9.5 years; 403 men) were included in this study. Examples of various morphologies of fQRS on ECGs are shown in Figure 1 . fQRS was present in 169 patients (36.2%), and MACE developed in 38 (8.1%; death 3, myocardial infarction 19, congestive heart failure 11, and percutaneous coronary intervention before vascular surgery 5).
Clinical, echocardiographic, and electrocardiographic characteristics according to the presence or absence of perioperative MACE are shown in Table 1 . The proportions of patients with an age ≥70 years, previous history of ischemic heart disease and congestive heart failure, current smoking, and a positive myocardial perfusion SPECT result were significantly greater in patients with perioperative MACE than in those without. ST-T wave change and left ventricular hypertrophy by ECG were more common in patients with MACE. Left ventricular ejection fraction was significantly lower and serum creatinine level was significantly higher in patients with MACE. Mean RCRI and fRCRI were significantly greater in patients with MACE. However, no significant differences were found between patients with or without MACE in terms of gender, histories of hypertension, diabetes, hyperlipidemia, and cerebrovascular disease, body mass index, and Q wave and atrial fibrillation by ECG.
| Variable | All Patients (n = 467) | MACE | p Value | |
|---|---|---|---|---|
| Negative (n = 429) | Positive (n = 38) | |||
| Age (yrs) | 69.4 ± 9.5 | 69.2 ± 9.6 | 71.7 ± 7.4 | 0.117 |
| Age ≥70 yrs | 253 (54%) | 226 (53%) | 27 (71%) | 0.029 |
| Men | 403 (86%) | 372 (87%) | 31 (82%) | 0.378 |
| Body mass index (kg/m 2 ) | 22.5 ± 3.1 | 22.5 ± 3.2 | 22.0 ± 2.9 | 0.326 |
| Hypertension | 273 (59%) | 250 (58%) | 23 (61%) | 0.787 |
| Diabetes mellitus | 161 (35%) | 143 (33%) | 18 (47%) | 0.081 |
| Hyperlipidemia | 63 (14%) | 58 (14%) | 5 (13%) | 0.946 |
| Previous ischemic heart disease | 71 (15%) | 61 (14%) | 10 (26%) | 0.047 |
| Previous congestive heart failure | 29 (6%) | 22 (5%) | 7 (18%) | 0.006 |
| Previous cerebrovascular disease | 66 (14%) | 58 (14%) | 8 (21%) | 0.201 |
| Current smoking | 280 (60%) | 264 (62%) | 16 (42%) | 0.017 |
| Creatinine (mg/dl) | 1.3 ± 1.5 | 1.2 ± 1.2 | 2.5 ± 3.3 | 0.020 |
| Creatinine ≥2 mg/dl | 35 (8%) | 26 (6%) | 9 (24%) | 0.001 |
| Left ventricular ejection fraction (%) | 56.8 ± 8.5 | 57.2 ± 7.8 | 51.7 ± 13.3 | 0.022 |
| Left ventricular ejection fraction ≤50% | 52 (13%) | 39 (11%) | 13 (37%) | <0.001 |
| Pathologic Q waves | 49 (11%) | 42 (10%) | 7 (18%) | 0.101 |
| Atrial fibrillation | 42 (9%) | 35 (8%) | 7 (18%) | 0.067 |
| Right bundle branch block | 27 (6%) | 25 (6%) | 2 (5%) | 1.000 |
| Left bundle branch block | 3 (1%) | 3 (1%) | 0 (0%) | 1.000 |
| ST-T changes | 66 (14%) | 52 (12%) | 14 (37%) | <0.001 |
| ST depression | 38 (8%) | 29 (7%) | 9 (24%) | 0.002 |
| T wave inversion | 49 (11%) | 40 (9%) | 9 (24%) | 0.011 |
| Left ventricular hypertrophy | 89 (19%) | 76 (18%) | 13 (34%) | 0.013 |
| QRS duration (ms) | 93.6 ± 16.0 | 93.5 ± 16.1 | 95.2 ± 15.4 | 0.520 |
| Fragmentation of QRS | 169 (36%) | 147 (34%) | 24 (63%) | <0.001 |
| Positive myocardial SPECT | 100 (21%) | 82 (19%) | 18 (47%) | <0.001 |
| RCRI score | 0.8 ± 0.9 | 0.7 ± 0.9 | 1.4 ± 1.2 | 0.003 |
| fRCRI score | 1.1 ± 1.1 | 1.1 ± 1.0 | 2.0 ± 1.4 | <0.001 |
fQRS was more frequent in patients with perioperative MACE. The sensitivity, specificity, positive predictive value, and negative predictive value of fQRS for predicting perioperative MACE were 63.2%, 65.7%, 14.2%, and 95.3%, respectively. By multivariate logistic regression analysis, fQRS (odds ratio 2.830, 95% confidence interval 1.378 to 5.813) was an independent predictor of perioperative MACE after adjusting for RCRI ( Table 2 ).
| Variable | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Diabetes mellitus | 1.513 | 0.738–3.103 | 0.258 |
| Previous ischemic heart disease | 0.965 | 0.384–2.429 | 0.940 |
| Previous congestive heart failure | 3.134 | 1.065–9.226 | 0.038 |
| Previous cerebrovascular disease | 1.423 | 0.590–3.434 | 0.433 |
| Creatinine ≥2 mg/dl | 3.118 | 1.227–7.925 | 0.017 |
| fQRS on ECG | 2.830 | 1.378–5.813 | 0.005 |
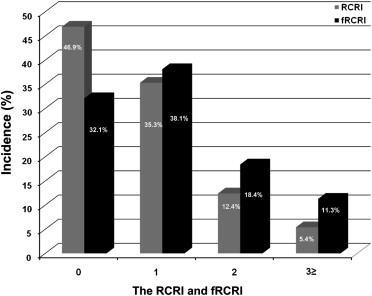
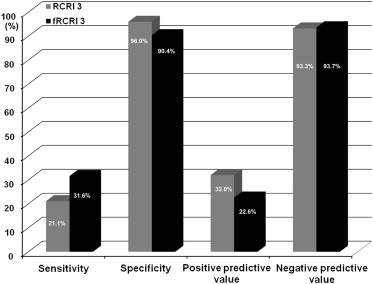
The perioperative cardiac risks in our cohort as defined by RCRI included diabetes (n = 161, 34.5%); renal insufficiency (n = 35, 7.5%), and previous histories of ischemic heart disease (n = 71, 15.2%), congestive heart failure (n = 29, 6.2%), and cerebrovascular disease (n = 66, 14.1%). Mean RCRI was 0.8 ± 0.9, and the incidences of RCRI 0, 1, 2, and ≥3 were 46.9% (n = 219), 35.3% (n = 165), 12.4% (n = 58), and 5.4% (n = 25), respectively ( Figure 2 ). When fRCRI data were analyzed, mean fRCRI was 1.1 ± 1.1, and the incidences of fRCRI 0, 1, 2, and ≥3 were 32.1% (n = 150), 38.1% (n = 178), 18.4% (n = 86), and 11.3% (n = 53), respectively. RCRI and fRCRI distributions were significantly different (p <0.001), and 28 patients (48.3%) originally classified as RCRI 2 were reclassified as fRCRI 3. Interestingly, fRCRI 3 was found to predict perioperative MACE more sensitively than RCRI (10.5% higher; Figure 3 ).