Chapter 1 A History of Vascular Surgery
Prologue
To place the Hunters in a clear perspective in regard to nonmedical history, one should note that they were contemporaries of George Washington and Benjamin Franklin. William Hunter was born in Scotland in 1718, his brother John was born 10 years later; William died in 1783, and John died in 1793.1,2 John was even made a member of the American Philosophical Society, although he never attended a meeting.
William Hunter preceded John to London, where he soon established a busy medical practice and interested himself in many subjects, including aneurysms. In fact, William proposed the concept that a lancet used carelessly during bloodletting might enter both artery and vein, and after healing, the two channels might be connected. He thus imagined an arteriovenous fistula. He soon found just such a patient and described the clinical manifestations with great accuracy.3 William’s primary activity, however, was focused on obstetrics and on the teaching of anatomy. John became his assistant in this latter project.
John Hunter is remembered for many things, but especially for his studies of the dynamics and efficiency of collateral arterial circulation, which he described in the vessels feeding the antlers of a stag after he had interrupted the major arteries in its neck. More renown came from his ligation of the femoral artery in its subsartorial course at a distance above a popliteal aneurysm—in Hunter’s canal.1,2
To be sure, others had preceded him in performing proximal ligation of arteries to treat aneurysms. In the third century, a Roman surgeon named Antyllus had described proximal and distal ligation of the artery, followed by incision of the aneurysm and removal of its contents—a formidable operation without either anesthesia or asepsis.4 In 1680, Purmann, faced with a large aneurysm in the antecubital space, performed ligation of the vessels and excision of the aneurysmal mass.5 In 1714, Anel described an operation in which he placed one ligature on the artery at the proximal extent of the aneurysm. Hunter, however, had found that the ligature would sometimes cut through the artery when it was placed too close to the popliteal aneurysm; therefore he chose a site that was more remote, but was easily reached by the surgeon and would preserve collaterals. Most of Anel’s patients suffered from false aneurysms caused by bloodletting in otherwise healthy arteries. The femoropopliteal aneurysms treated by Hunter were due to degenerative processes, probably a mixture of syphilis and trauma.1,6
Many other surgeons were ligating aneurysms in various anatomic sites at this time. Cooper, one of John Hunter’s students, was soon established as one of the early vascular surgeons when he ligated the carotid artery for an aneurysm in 1805,7 as well as the aorta for an iliac artery aneurysm.8 Only these few important events occurred before the latter part of the nineteenth century.
At the time, ligation was virtually the only procedure available to surgeons for the management of arterial problems, and those problems were limited to the control of hemorrhage and the treatment of aneurysms. Hallowell in Newcastle-on-Tyne performed one arterial repair of an artery torn during bloodletting. The laceration was a short one, and at the suggestion of Lambert, he placed a short (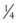 inch) steel pin through the edges of the wound and looped a ligature around it in a figure-of-eight pattern, approximating the edges of the wound with apparent success. Hallowell wrote to William Hunter concerning this operation in 1761, foreseeing that if this were a successful technique, “we might be able to cure wounds of some arteries that would otherwise require amputation, or be altogether incurable.”9 That Hallowell wrote to William instead of John is probably due to William’s published work on arteriovenous fistulas secondary to inept bloodletting. Twelve years later in 1773, Asman reviewed the Newcastle repair, attempted some experiments of his own that were disastrous, and concluded that such a procedure could not work and that Lambert and Hallowell’s efforts had probably failed as well.10 After Asman’s criticism, the matter of arterial repair rested quietly for nearly another 100 years.
inch) steel pin through the edges of the wound and looped a ligature around it in a figure-of-eight pattern, approximating the edges of the wound with apparent success. Hallowell wrote to William Hunter concerning this operation in 1761, foreseeing that if this were a successful technique, “we might be able to cure wounds of some arteries that would otherwise require amputation, or be altogether incurable.”9 That Hallowell wrote to William instead of John is probably due to William’s published work on arteriovenous fistulas secondary to inept bloodletting. Twelve years later in 1773, Asman reviewed the Newcastle repair, attempted some experiments of his own that were disastrous, and concluded that such a procedure could not work and that Lambert and Hallowell’s efforts had probably failed as well.10 After Asman’s criticism, the matter of arterial repair rested quietly for nearly another 100 years.
John Hunter’s less widely known contributions are scattered throughout the immense museum he left to the Royal College of Surgeons of England, and they hint at an understanding of arterial pathology that would not be general knowledge for half a century. They include dissections of several atherosclerotic aortic bifurcations (specimens P.1177 and P.1178), showing the atheromatous lesion at the aortic bifurcation that Leriche would describe 150 years later; a carotid bifurcation with an ulcerated atheroma from a patient who died of a ruptured syphilitic thoracic aneurysm (specimen P.1171); and an extracranial internal carotid aneurysm (specimen P.282) in a patient whose neatly described symptoms are almost typical of what today are recognized as classic transient ischemic episodes.11 Regrettably, most of Hunter’s notes did not survive to provide more than this fragmentary view of his understanding of vascular disease. To cap it all, in a postmortem specimen, Hunter had dissected the atheromatous layers (although the term atheroma had not yet come into use) from the remaining intact wall of an atherosclerotic terminal aorta (specimen P.1176), foreshadowing dos Santos by 150 years.
Both Hunter and Cooper seemed to hold with the teleologic belief of the times that when senile or spontaneous gangrene occurred in older persons, thrombosis of the major vessels supervened so that the patient would not bleed to death when the gangrenous part separated.12 It was Cruveilhier who first clearly stated that the phrase “gangrene due to obstruction of the arteries” by thickening and by thrombosis should replace the terms spontaneous and senile gangrene,13 but he attributed the concept to Dupuytren.
The recognition that arterial obstruction causes functional disability that limits the use of the affected part may have arisen in the veterinary world. Bouley described the clinical picture in a horse in 1831.14
Four years later in 1835, a nearly anonymous physician on the ward of a Professor Louis provided the first clear description of human claudication. Barth’s patient was a 51-year-old woman who died of heart failure resulting from mitral valvular disease. His report described her incidental history of claudication in terms that we would recognize today.15 In the postmortem report, he noted thrombosis of the terminal aorta and included a sketch suggesting that the lesion was a thrombosed hypoplastic terminal aorta, a contracted atherosclerotic lesion, or a combination of both. Barth also repeated Hunter’s observation that the obstructing material could be separated easily from the residual intact arterial wall. Barth was never identified further, not even by an initial.
Charcot is often erroneously given credit for recognizing the syndrome of intermittent claudication caused by arterial insufficiency in humans.16 Charcot described, just as Bouley had done, the vanishing pulses, the cold extremity, and what is now recognized as the loss of sympathetic tone in a horse in the throes of a spasm of severe claudication; he reported a human case as well. Homans liked to joke that Charcot observed the former because he spent so much time at the horse races.
Successful Arterial Suture
Such information was of little utility to surgeons, however, until arterial repair became a reality. Consistent with the observations of Asman, several German masters had deemed arterial repair (as opposed to ligation) to be impossible. Langenbeck stated in 1825 that, because the primary requirement for healing is perfect rest, an arterial incision could never heal as long as the pulsatile movements of the arterial wall continued.17 Heinecke was certain that the patient would bleed to death through the suture holes and the apposed edges of the arterial wall.18
Repair of small injuries to veins, however, was becoming an established procedure. The lateral ligature, in which a clamp is placed on the defect in the venous wall and a ligature is tied around the puckered wall, had been performed in 1816.19 The first lateral suture of a venous defect (an erosion of the common jugular vein from an infected neck wound) was undertaken by Czerny in 1881, but the patient died of sepsis and hemorrhage.20 Jassinowsky19 credits Schede21 with the first successful repair of a large venous injury (to the common femoral vein) by lateral sutures.
Going beyond the stage of venous repair, Eck reported the experimental creation of a portocaval fistula in dogs.22 The original description hints that he had little to confirm his success. Among a series of eight dogs, one died within 24 hours, six lived 2 to 6 days, and the one survivor “tired of life in the laboratory and ran away after 2 months.” The doctoral dissertation of Jassinowsky, written in 1889 and based purely on library research, reviewed the published information on arterial suture and concluded that it could not be successful at that time, but that there might be hope in the future.19
Only 2 years later, however, Jassinowsky himself succeeded. In 1891, he reported his successful animal experiments involving arterial suture.23 The suture he described was passed carefully only two thirds of the way through the media; he tried to avoid penetrating the intima, except in very thin-walled vessels. This effort should be recognized for its intrinsic difficulty using even the finest milliner’s needles, because without sutures swaged onto needles, two pieces of suture have to be dragged through the arterial wall. Dörfler modified Jassinowsky’s method and passed the suture through all thicknesses of the arterial wall.24 He also recognized that the arterial suture exposed in the lumen of the vessel did no harm if uninfected. He observed that it soon became covered with a glistening membrane. Shortly thereafter in 1896, Jaboulay and Briau described successful end-to-end carotid arterial anastomoses in animals using an everting U-shaped suture.25
Jaboulay was one of the surgeons in Lyon, France, under whom Carrel studied. When Sadi Carnot, the president of the Republic of France, was wounded by an assassin and died because no one dared to try to repair his portal vein, Carrel was highly critical, because he believed that blood vessels could be sutured as well as any other tissue.26 He soon undertook experimental arterial anastomoses; some of the earliest of these were arteriovenous communications in which the high-flow system ensured patency. Carrel’s contributions to technical arterial surgery included methods that vascular surgeons routinely use today.27,28 He devised the triangulation suture to facilitate end-to-end anastomosis, described the patch technique to anastomose a small vessel to the side of a larger one (as in replantation of an inferior mesenteric artery), and pioneered the use of vessel grafts and organ transplantation. His work, however, was not fully accepted in the United States for many years. In part, this stemmed from disputes that arose between him and Guthrie, who was his coworker for 1 year.29
In contrast, European surgeons not only accepted Carrel’s work but also began to follow his lead. In 1906, Goyanes of Madrid, Spain, resected a popliteal aneurysm, then restored arterial continuity with an in situ venous graft using the popliteal vein, which was probably the first successful clinical vascular replacement.30
Surgeons in the United States were beginning to perform vascular surgery in their own way. In New Orleans in 1888, Matas described a landmark operation.31 He stumbled onto the surgical procedure for which he is commonly remembered, endoaneurysmorrhaphy, when an aneurysm for which he had ligated only the proximal brachial artery, with apparent initial success, began to pulsate again 10 days later. Reportedly, it was a medical student who called this to the professor’s attention. He chose to reoperate and to ligate the brachial artery distally. Even after this distal ligation, the aneurysm continued to pulsate, and he was forced to open the aneurysm, clean out the sac (the operation performed by Antyllus), and oversew the other arteries feeding the aneurysm from inside the sac. This foreshadowed the problems with endoleaks that confound vascular surgeons who place endovascular aortic prostheses today.
Matas’s operation differed from that of Antyllus, in that Matas used a suture within the aneurysmal sac to obliterate the feeding vessels instead of ligating them outside the sac. The extensive dissection that would have been required outside might have damaged the collateral circulation and other adherent anatomic structures. It was many years before Matas performed another endoaneurysmorrhaphy, because most patients were treated successfully by simple proximal ligation.32 Matas ultimately expanded the descriptions of his technique to include “restorative” and “reconstructive” modifications, and he reported an approach to the arteriovenous fistula through the venous component,33 as had been proposed by Bickham.34
Murphy, of Chicago, performed a series of experiments on animals in which he successfully restored continuity by invagination of the proximal into the distal vessel. In 1897, he presented a successful human case.35 Edwards briefly revived this anastomotic technique of invagination when he recommended the use of the first braided nylon grafts.36
Murphy’s invagination techniques were reflected in other nonsuture methods of anastomosis: Nitze37 and Payr38 used small metal or ivory rings through which the vessel was drawn, everted, and tied in place; this unit was then inserted into the mouth of the distal vessel, and another ligature secured it there. This is substantially the Blakemore tube,39 used during World War II, albeit without signal success.40
During his tenure at Johns Hopkins Hospital, W.S. Halsted had an abundance of traumatic and syphilitic aneurysms commanding his attention. In the early 1900s, Carrel visited Halsted and described his own technical experiments, including his early arteriovenous anastomoses. As a result, Halsted almost made history in 1907 when he faced the dilemma of a patient whose popliteal artery and vein had been sacrificed during an en bloc dissection of a sarcoma of the popliteal space.41 Halsted went to the other leg, took the saphenous vein, reversed it, and anastomosed the distal saphenous vein to the proximal femoral artery. For his distal anastomosis, however, he chose the popliteal vein. Although the graft pulsated for 40 minutes, it soon thrombosed. It is possible that Halsted was pursuing the chimera of reversal of arterial flow through the venous bed. One can only imagine what a dramatic leap forward vascular surgery would have made if Halsted, with his superb supporting cast of talented surgeons, had chosen the popliteal artery for the distal anastomosis and had achieved a truly successful arterial reconstruction in the pattern of the modern vascular surgeon.
There is considerable literature on attempts to revascularize ischemic extremities via arteriovenous anastomoses. San Martín42 and A. E. Halsted43 attempted to improve the distal circulation using arteriovenous anastomoses.
Meanwhile, German surgeons such as Höpfner,44 Lexer,45,46 and Jeger47 had become familiar with the use of short (<10 cm) vein grafts. Höpfner described the bypass procedure, which was illustrated in an encyclopedic book by Jeger. Jeger’s book, republished posthumously in 1937, included a foreword that described Jeger’s replantation of the completely severed arm of a German soldier, which he had performed in 1914. One year later, Jeger came to an untimely death from typhus while on the Russian front.
Lexer collected and reported on 65 vein transplants, 13 of which were his personal cases.45 In 8 of these 13 cases, Lexer had obtained a distal pulse. This report prompted a Polish surgeon, Weglowski, to present his own personal series of 51 vein grafts, mostly for trauma, operated on between 1914 and 1921; in 40 patients he could document good distal pulses and normal arterial tracings.48 Yet all this seemed to be forgotten for the next 25 years as Germany suffered the agonies of the interbellum years, and as the forceful and charismatic personality of Leriche appeared on the scene (Leriche’s role is described in a later section).
Abdominal Aortic Aneurysms
Vesalius is said to have been the first to describe an abdominal aneurysm.49 The successful management of the abdominal aneurysm is certainly one of vascular surgery’s major accomplishments. The technical maneuvers described previously concerning the ligation of aneurysms in various anatomic sites usually involved aneurysms of the peripheral vessels; aneurysms of the trunk were sacrosanct, because proximal control was not feasible. Cooper had continued many of Hunter’s studies, including evaluation of collateral arterial supplies. In 1805, he had ligated the common carotid artery for an aneurysm,7 but he opened the door for even wider surgical applications when, in 1818, he ligated the abdominal aorta to control external hemorrhage from an aneurysm of the external iliac artery that had eroded to the surface of the skin of the flank, bleeding openly at that site.9
Interest in the treatment of major vessel aneurysms lagged for almost a century. Eventually Colt, at the end of the nineteenth century, used wire to pack an aneurysm and then heated the wire.50 Blakemore and King revived interest in this technique in 1938,51 and many surgeons undertook modifications of the wiring technique, largely without success. Meanwhile, more direct attempts were being made by the major actors in the next scene: Matas of New Orleans and Halsted of Baltimore. Their interest in the management of vessel trauma, and in the management of late sequelae of such trauma, provided material for the fertile imaginations of the many surgeons who were emboldened to follow in their footsteps. Reid reported the experience of the Johns Hopkins Hospital (headed by Halsted) with aneurysms in 1926.52 The aneurysms treated included many varieties, both anatomic and etiologic, but treatment of abdominal aneurysms was substantially a failure. These operations were only preparation for the end of ligation as a treatment for aneurysms of the abdominal aorta.
Matas finally accomplished a successful aortic ligation (just below the renal arteries) for an aneurysm at the bifurcation of the aorta. He reported it first in 1925 and then again in 1940.53,54 In the issue of Annals of Surgery that contained Matas’s second report was a similar paper by Elkin,55 as well as a hint of the coming era of vascular reconstruction in a report by Bigger of Virginia.56 Bigger had ligated the neck of an abdominal aneurysm using fascia that he expected to loosen gradually and allow restoration of flow. With the protection of this temporary control, he performed a plication of the aneurysm, restoring the aorta to its proper caliber. The patient had a protracted survival without recurrence of the aneurysm and also with restoration of femoral pulses.
About this time, however, cardiac surgery began to emerge. During the first decade of the twentieth century, Jeger had proposed valved venous grafts between the left pulmonary veins and the left ventricle to bypass mitral stenosis, and a valved venous graft from the left ventricle to the innominate artery to bypass aortic stenosis.47 In the mid 1920s, Cutler and colleagues57 had attempted to treat mitral stenosis surgically, but with minimal success. A valvulotome was used through a ventricular approach.
Nonetheless, the influence of these attempts led Gross to the successful ligation and, 5 years later, division of the patent ductus arteriosus.58,59 In Baltimore, Blalock and Taussig60 began their series of pioneering surgical procedures for various cardiac anomalies, the first and most dramatic of which was the “blue baby” operation—the creation of a systemic shunt from the subclavian artery to the pulmonary artery in patients with congenital pulmonic stenosis.
Crafoord and Nylin61 reported the successful end-to-end anastomosis of the aorta after resection of an aortic coarctation at the same time that Gross and Hufnagel62 carried out their first case. This last operation demonstrated that lesions of the thoracic and abdominal segments of the aorta were amenable to a surgical approach.
Development of Vascular Prostheses
Following the experience in the laboratory reported by Abbe,63 Tuffier had used rigid tubes of metal and of paraffined glass to try to replace small- to medium-size arteries during World War I, without success.64 Similar tubes were used in World War II, but the results were no better than those obtained by immediate ligation of the artery.40 Hufnagel chose a more inert surface, methylmethacrylate, as well as a tube with a better hemodynamic design.65 Hufnagel’s tubes functioned remarkably well in animal experiments, except for the difficulty in securing them within a major artery such as the aorta without the risk of ultimate erosion. Eventually the use of pliable plastic fabrics virtually eliminated the rigid tube.
In 1947, Hufnagel reported on the use of rapid freezing for the preservation of arterial homografts and suggested their utility in the repair of long aortic coarctations.66 Gross, who at first feared that frozen vessels could not survive, published a laboratory and clinical report on his experiences with homografts preserved in electrolyte solutions for use in various cardiac operations, but particularly for the management of coarctation of the aorta.67 Swan soon used a homograft for a thoracic aneurysm associated with a coarctation.68
The arterial homograft initially seemed to be a good substitute for the thoracic or abdominal aorta. At first, fresh grafts were used; then they were preserved in Tyrode’s solution. Improvements in the preservation of grafts by freezing69 and then lyophilization70 facilitated the development of arterial graft banks. Early successes were soon erased by late failures of the homografts, however, and a truly satisfactory aortic substitute was sorely needed.
In 1952, Voorhees and colleagues observed that fabric threads in a chamber of the heart soon became covered with endothelium.71 Dörfler had made a similar gross observation 60 years earlier, but had not carried the observation to its conclusion.64 Voorhees and associates at Columbia pursued experiments not only with Vinyon-N, but also with parachute silk and other materials. Many fabrics were tried, and most were quickly discarded. Braided and crimped nylon tubes were introduced by Edwards and Tapp,36 but it was soon discovered that nylon rapidly lost strength and was unsatisfactory.72 Both Orlon73 and Teflon74 were used. Szilagyi and colleagues75 and Julian and colleagues76 introduced various fabrications of Dacron. The transcripts of the vascular surgery meetings of the late 1950s might be mistaken for a textile journal, as various weaves, deniers, calenderizing, and the advantages of braid versus knit versus taffeta weaves were discussed. The summation of the principles of vascular grafting by Wesolowski and coworkers had enunciated the importance of porosity,77,78 but the substantially nonporous Teflon undercut that thesis.
The knitted Dacron introduced by DeBakey and colleagues placed a generally successful graft in the hands of every surgeon.79 Subsequent modifications by the addition of velour to the surface by Sauvage80 and also by Cooley81 refined this outstanding contribution. Wesolowski and colleagues’ concept78 that the fabric tube would become “encapsulated” and might develop a firm new endothelial surface has been pursued as a goal but has not been achieved in humans.
The immediate porosity of the grafts has been troublesome on occasion, especially in patients who require heparinization or in whom even minor blood loss from a weeping graft is intolerable. Impregnation with either collagen82 or albumin83 was a useful advance. Teflon in the form of an extruded tube (Gore-Tex) rather than as a woven or knitted fabric was introduced clinically by Soyer,84 and it has achieved great popularity. Introduced first for use as a venous substitute, it came to be used extensively in arterial reconstructions as a second choice after autologous vein,85 although Quiñones-Baldrich and colleagues86 expressed a preference for Gore-Tex in femoral anastomoses above the knee, preserving the vein for more distal reconstructions if such become necessary.
Biological substitutes other than the arterial homograft have also been suggested. Rosenberg and associates used bovine carotid arteries that had been subjected to enzymatic treatment to remove all the tissue-specific protein, except the basic structural collagen of the bovine artery.87 Sawyer and colleagues88 attempted to modify the bovine heterograft by inducing a negatively charged lining in an effort to inhibit thrombosis. Dardik and coworkers89 used treated umbilical vein grafts supported with a mesh of Dacron as a peripheral arterial substitute.
Modern Management of Aortic Aneurysms
The grave risk posed by abdominal aneurysms was exposed in a timely paper by Estes in 1951.90 Other experiences with the aorta were preparing the way for present-day management of abdominal aneurysms. Alexander and Byron91,92 had resected a thoracic aneurysm associated with coarctation of the aorta and successfully oversewn the ends of the vessel, although the patient ultimately died of renovascular hypertension. Swan had used a homograft to replace a thoracic aneurysm.68
Various attempts were made to use either reactive cellophane93 or the tissue-irritating plasticizer dicetyl phosphate94 as a means of inducing sclerosis that might restrain the dilatation of the aneurysm. These attempts to control the growth of the aneurysm were not rewarding.
Oudot95 set the stage for other forms of aortic replacement when he used a homograft to restore circulation in a patient with Leriche syndrome. Dubost is recognized as the pioneer who first successfully replaced an abdominal aneurysm with a homograft on March 19, 1951.96 Schaffer and Hardin97 actually preceded Dubost by 4 weeks, but their publication appeared considerably later and focused on the use of a polythene shunt to maintain distal circulation during the operation rather than on the priority of resecting the aneurysm itself. It appears that Wylie actually accomplished a successful endarterectomy of an abdominal aneurysm on January 13, 1951. Similarly, Freeman and Leeds treated three patients, two successfully, with inlay grafts of the patient’s own iliac veins beginning on February 12, 1951. Wylie’s and Freeman’s operations were not graft replacements, however, but rather modifications of Bigger’s procedure.56
Dubost’s operation was soon followed by those of Julian,98 Brock,99 DeBakey,100 and Bahnson.101 It is a curious twist of fate to find that Dubost had left the practice of colorectal surgery to become a cardiac surgeon after he saw Blalock and Bahnson perform dramatic cardiac operations while they were visiting France in the late 1940s. Szilagyi’s102 classic study of the benefits of the operation in 1966 provided confirmation and justification of the thesis Estes had presented in 1950.
The complicated abdominal aneurysm still posed a major problem. Ellis was one of the first to implant the renal arteries into the graft when the aneurysm was found to include their orifices.103 Etheredge104 extended this operation to resect a major thoracoabdominal aortic aneurysm. He used a heparinized plastic shunt of the type described in Schaffer’s resection and replacement of an abdominal aneurysm with a homograft in March 1951. Etheredge established the shunt, divided the aorta, and performed the proximal anastomosis; he then moved the clamp down the graft after each successive visceral anastomosis was completed and finished with the lower aortic anastomosis to the graft.
DeBakey and colleagues105 reported in 1956 a series of complicated abdominal and thoracoabdominal aneurysms that were resected with a technique similar to that later used by Shumacker.106 In 1973, Stoney and Wylie107 popularized the long thoracoabdominal incision for the approach to this lesion. The great advance in the management of these complicated lesions was made by Crawford,108 who introduced a direct approach to the aneurysm in which the aorta is clamped above and below and then opened throughout the length of the aneurysm. A fabric graft is sewn into the proximal aorta; the major groups of arteries, including the lower intercostals when possible, are sewn into the wall of the fabric tube using the expeditious Carrel patch method of anastomosis; then the distal anastomosis is completed. This direct method has greatly simplified the approach to these challenging lesions.
The placement of a graft within the lumen of an aneurysm—whether abdominal, thoracic, or peripheral—was logically extended by a technique that allows one to place the graft within the aneurysm from a distance through a short arteriotomy in either the femoral or the external iliac artery. The evolution of this method stems circuitously from Dotter and coworkers.109 In 1983, they attempted to improve the results of simple arterial dilatation or to maintain the patency of a graft with small endarterial spiral coils. After several generations of devices that did not gain wide acceptance, Palmaz and associates110 introduced a metal mesh stent that can be expanded by balloon dilatation, which secures the stent in place. Introduced originally to maintain the patency of a segment of artery that had undergone percutaneous dilatation, this method was at first used in occlusive disease, but Parodi and colleagues111 modified the technique to secure a fabric graft that had been placed within an aneurysm. Although initially used as a tube graft, modifications soon allowed the placement of bifurcation grafts.112,113 The anticipated decrease in morbidity and mortality accompanying this method led to its widespread use, although not all aneurysms are amenable. The need for prolonged follow-up versus the security of a one-time operation has raised the clinical question of the ultimate role of the endovascular repair of aneurysms. Here the narrative becomes so contemporaneous as to require clinical rather than historical description.
Peripheral Arterial Aneurysms
In 1949, Linton used Leriche’s concept of arteriectomy and sympathectomy for the management of 14 patients who had popliteal aneurysms—an ingenious approach that resulted in no amputations in his series.114 The patients received a preliminary sympathectomy; shortly afterward, or sometimes at the same operation, the aneurysm was resected, with ligation of the artery above and below it.
The ability to replace vessels of the size of the popliteal artery brought to the fore the concept that the popliteal aneurysm had a risk-benefit pattern similar to that of the abdominal aneurysm. If operations were done electively, the results were excellent, but once thrombosis occurred, the risk to the limb was grave, as Wychulis and associates demonstrated.115 Wylie (in the discussion of Wychulis115) and Edwards116 introduced the procedure of excluding the aneurysm and restoring flow through a bypass technique.
Occlusive Arterial Disease
As mentioned earlier in this chapter, it was not until the middle of the nineteenth century that the relationship between arterial occlusion and gangrene was clearly established. Repair of acute injuries had been accomplished, but management of more chronic arterial obstructions had hardly been considered a surgical problem. Recognition of the clinical symptoms of less severe ischemia came to surgery by way of veterinary medicine.14 The association between the sympathetic nervous system and the arteries was recognized in the early twentieth century, especially during World War I.
Leriche, born in 1879, had been educated and trained at Lyon, where he had known Jaboulay and Carrel. Shortly after Leriche completed his training, World War I broke out, and Leriche acquired considerable experience with wounds of the extremities. After he was demobilized, Leriche continued to work in a trauma hospital in Lyon for several years. There he saw many patients with posttraumatic neuralgias, and he developed his concepts of the role of the sympathetic nervous system and the possible treatment by periarterial sympathectomy, about which he had first written in 1917.117 Then, seeing patients with arterial thrombosis caused by artérite (a nonspecific term used by French surgeons to describe arterial disease and occlusion in general), Leriche concluded that if the patient was seen before the occluding thrombosis was too widespread, local resection of the thrombosed artery provided relief. Because many patients did well after this simple procedure and soon developed relatively warm feet, he concluded that the collateral circulation in these patients must have been satisfactory and that the coldness of the extremity was due to vasospasm rather than insufficient arterial flow. He therefore applied the principle of sympathectomy, first as a periarterial operation, then as an arteriectomy (excising the obstructed segment), and then as a division of the sympathetic rami.118
Diez,119 dissatisfied with the results of periarterial sympathectomy, modified that operation into the lumbar ganglionectomy. At nearly the same time, Royle120 and Hunter121 introduced the same fruitless operation for the management of spasm in striated muscle. Use of this operation for the management of pain syndromes and ischemic extremities remains controversial.
One of Leriche’s most important early observations was the definition of the syndrome that now bears his name, the atherosclerotic obliteration of the terminal aorta and the iliac arteries. He described this in 1923, during the period when he was beginning to evaluate arteriectomy.122 It would be 17 years, however, before he found a suitable case in which he could perform resection of the aortic bifurcation and lumbar sympathectomy.123 Leriche’s surgical clinic became famous, and he attracted a long line of surgeons who came to learn: DeBakey, Learmonth, dos Santos, and Kunlin, to name a few.
The possibility of effective arterial suture anastomosis had been developed through the ideas of Jaboulay25 and Carrel27 at Lyon. After World War I, another surgeon from Lyon assumed a major role in vascular surgery.
In 1909, Murphy removed an embolus from the common iliac artery and restored flow into the femoral system. Although locally successful, distal thrombosis required a distal amputation.124 Two years later, Labey (as cited by Mosny and Dumont125) removed an embolus from the artery of a patient, with complete success. Embolectomy was thereafter performed with occasional success worldwide, but it did not become a fully satisfactory procedure because of the need to operate hastily, before extensive distal thrombosis supervened. After the clinical introduction of heparin by Murray,126 it became possible to extend the indications for embolectomy and to extend the time limit for undertaking the procedure and thus improve the results.
Surgeons such as João Cid dos Santos and his father, Reynaldo, used heparin to prevent thrombosis after performing the nearly forgotten Matas endoaneurysmorrhaphy.127 The younger dos Santos believed that with the protection of heparin, he might be able to remove chronically adherent arterial emboli and their associated thrombus and achieve healing without rethrombosis. After finding such a patient with advanced renal disease and a seriously ischemic extremity, dos Santos removed the clot and reestablished flow. He was chided by the pathologist for having removed the intima as well. After another successful case, in which he removed a chronic thrombosis of the subclavian, axillary, and brachial arteries secondary to scalenus anticus syndrome, he sent his report to Leriche. Leriche presented the work in the name of dos Santos to the French Academy of Surgery128 and introduced endarterectomy to the surgical world. It is interesting to note that neither of these patients suffered primarily from the usual forms of atherosclerotic thrombosis.
Subsequently, Freeman and colleagues,129,130 Wylie and associates,131 and others adopted the operation, using the open technique that was championed primarily by Bazy and coworkers.132 In September 1951, Wylie described endoaneurysmectomy and endarterectomy of the aorta. At the time, my colleagues and I had undertaken six procedures without success, but in the summer of 1951, Wylie had visited us, and in October 1951 we performed the first successful endarterectomy in our series.133 The operation consisted of a combination of the Matas endoaneurysmorrhaphy and the dos Santos endarterectomy (or the technique as revised by Reboul): an abdominal aneurysm was endarterectomized, tailored to a proper size, and wrapped with fascia lata, and an endarterectomy in continuity was performed throughout the length of the left iliofemoropopliteal system. In fact, these operations were only extensions of the aneurysm repair performed by Bigger in 1940.56
Cannon and Barker later introduced the long, closed endarterectomy using intraluminal strippers,134 which was a modification of the original method of dos Santos. Several similar varieties of endarterectomy loops were devised by Butcher135 and by Vollmar and Laubaeh,136 among others. A period of early success was followed by disenchantment owing to the difficulty of the operation in comparison with the increasingly popular grafting procedures.
Leriche and his close associate Kunlin had not had great technical success with endarterectomy, especially in the femoral artery system. Kunlin revived the use of the vein graft in the form of a long venous bypass.137 His first patient had already undergone arteriectomy and sympathectomy, thus justifying the then-unorthodox procedure.
Saphenous vein grafting was useful only in the femoral and iliofemoral systems, however, and it remained for Oudot to perform a comparable reconstructive operation on the aorta using an aortic homograft,95 which thoracic and cardiac surgeons were already using to replace segments of the thoracic aorta. Oudot was presented with a 51-year-old patient with claudication as a result of proximal iliac and distal aortic occlusion. Oudot’s operation is commonly described as a simple bifurcation graft, common iliac to common iliac, but it was actually a much more complicated procedure. He approached the bifurcation extraperitoneally through a left flank incision and resected the bifurcation. The patient’s internal iliacs were found to be thrombosed and were ligated. The external iliac arteries of the graft were very small, but the graft’s internal iliacs were large; Oudot therefore anastomosed the graft’s internal iliacs to the patient’s external iliacs. However, he did the left-sided anastomosis first and then found that the repaired vessel obstructed his view and hindered manipulations of the right-sided anastomosis. This difficult anastomosis thrombosed promptly. Oudot made the best of a bad situation and pointed out that he had done a perfect experiment, as there was still some discussion from Leriche’s camp about whether grafting at this level would be worthwhile. On the right side, Oudot had performed substantially nothing more than an arteriectomy; on the left, he had reconstituted the lumen. The right side was warm but pulseless and still fatigued easily, whereas the left side had a pulse and did not tire. Six months later, Oudot reoperated on the patient, who was still complaining of right-sided claudication; he performed an iliac-to-iliac “extraanatomic” bypass, as had been suggested by Kunlin in 1951.
The saga of the treatment of arterial disease continues with the development and then the failure of artery banks and the introduction of the plastic prosthesis, but by 1952 the stage was set for nearly everything that is done today. Linton’s espousal of the reversed saphenous vein in 1952 confirmed the approach of Kunlin and established the procedure of choice for peripheral reconstruction for many years.138
Endarterectomy did not die out completely; it persists in carotid operations, but only occasionally is it used in the aorta and as part of local tailoring procedures elsewhere. Edwards made one important attempt to use it in the femoral artery by means of a long patch; the procedure worked well unless the patch was so wide that it created a stagnant column of blood in the femoral artery.139 Femoropopliteal endarterectomy fell from favor because of its limited applicability to reconstructions that ended proximal to the distal portion of the popliteal artery. The full open repair was tedious, and most surgeons had limited success in restoring flow.
In recent years, however, closed endarterial procedures have become commonplace. Dotter and Judkins began in 1956 by using a stiff dilator,140 a procedure that was not widely accepted. Gruntzig and Hopff modified this method by using a balloon that could distend and fracture the stenotic plaque.141
Endarterial procedures have been extended to include not only dilatation and placement of emboli of several kinds in bleeding arteries, but also removal of atherosclerotic lesions by endarterial manipulations through a percutaneous route. A major requirement for endarterial procedures was believed to be endarterial visualization, beyond that provided by contrast radiography. Visualization began effectively with the work of Greenstone and others.142
Actual removal of plaque by several mechanical means followed: Simpson and associates143 used a side-biting forceps in a catheter, Kensey and coworkers144 used a catheter through which a rapidly rotating auger-like tip was passed, and Ahn and colleagues145 advocated a high-speed rotary bur. Others have used various forms of laser energy to destroy plaque.146 In one procedure, the laser recognizes the difference between plaque and normal arterial wall.147 In another, the laser-heated probe “melts” the atheroma.148 Further mechanical dilatation often accompanies these initial coring methods. Appraisal of these methods, however, belongs in the clinical rather than the historical section of this volume; they appear to achieve only limited removal of the atheromatous material and much less satisfactory results than the classic techniques of endarterectomy, albeit without requiring a major operative procedure.
Dotter and others109 proposed the addition of intraluminal stents to maintain graft patency, as well as the patency of vessels that had been dilated. In the surgical literature, this maneuver was largely ignored until Palmaz and associates110 introduced balloon-expandable stents, which were first used to maintain patency in dilated arteries. The use of percutaneous arterial dilatation and endarterectomy has suffered from inadequate and inconstant reporting standards in the hands of many nonsurgeons, but the technique appears to have reached a level of acceptance that requires the definition of its historical role.
Parodi and coworkers111 hybridized the technique of endarterial placement of these stents and added the placement of fabric grafts, a technique described previously in the section on aneurysms.
Two other important extensions of distal femoral reconstruction came on the scene. The first was introduction of the graft to the infrapopliteal artery. In 1960, Palma149 published descriptions of vein graft insertions into the tibial arteries. Later information from Palma (personal communication, 1990) indicates that these were performed as early as 1956. McCaughan described the exposure of the “distal popliteal artery” (more commonly known as the tibioperoneal trunk) and anastomoses to it in 1958,150 but his work went unrecognized because of his unconventional terminology. In that article, McCaughan151 described a successful graft into the tibial vessels in July 1957, using an exposure in the upper third of the calf. He presented six additional patients with grafts into the tibial segment in 1960. In 1966, McCaughan152 went one step further when he reported four grafts in which the distal insertion of the graft was into the posterior tibial artery at the ankle. Morris and coworkers153 and Tyson and DeLaurentis154 were other contemporary pioneers in the development of various configurations of infrapopliteal procedures.
The second extension of distal femoral reconstruction was application of the in situ vein graft, with destruction of valvular competence within the vein, by Hall.155 The procedure did not receive much attention until it was revitalized by Leather and associates in 1981.156 Many variations on the theme of the distal bypass have been introduced, combining free grafts and in situ methods.
Dardik and associates introduced the use of tanned human umbilical vein and then added a distal arteriovenous fistula.157 The fistula was not a revival of earlier attempts by Carrel and others to revascularize an extremity through the veins, rather it was an attempt to provide sufficient outflow for a long graft to ensure its patency, with some of the graft flow still directed through the distal arterial tree. DeLaurentis and Friedman introduced a method of sequential multiple bypasses in the extremity,158 and Veith and associates159 carried this to extremes with bypasses from one tibial artery to another, and even with bypasses beginning and ending below the malleolus. Nehler’s group160 applied this small vessel bypass technique to the management of small vessel disease in the distal upper extremity.
A different approach to the ischemic lower limb was advocated by Oudot and Cormier161 when they observed how frequently the superficial femoral artery was occluded, but the profunda femoris remained patent. Martin and coworkers described an extended form of profundaplasty, particularly as the site of insertion of a graft from above.162
None of these advances in reconstructive surgery has been helpful in the management of the frustrating syndrome of thromboangiitis obliterans, or Buerger’s disease. It is likely that von Winiwarter163 was describing the pathologic process of thromboangiitis obliterans, but his description and clinical correlation are ambiguous. Certainly, Buerger described the clinical picture,164 although neither he nor von Winiwarter noted the association with tobacco or the involvement of the upper extremities.
One other major contribution rounds out this section. In 1963, Fogarty and coworkers devised one of the most useful methods for managing occlusive arterial disease—the balloon embolectomy catheter for the extraction of clot in the treatment of embolization.165 This technique has been modified for use in many other arterial and venous operations and has even been adapted to many general surgical uses.



