Taxonomy of Paramyxoviridae virus
RSV, particularly, expresses two superficial glycoproteins: fusion (F) protein and binding proteins (G). These glycoproteins are very important in the virus lack of effectiveness and viral pathogenesis, and they are the main objectives of the host’s protective antibody production. G protein regulates the binding to the cell of the host. Then, F protein allows the binding of the plasma membranes of the virus and the host, which allows the virus to move into the cell. F protein also promotes the fusion of its plasma membranes, through which the virus can be transmitted from one cell to another. An interesting note is that these “syncytia” are rarely seen in vivo, but they are commonly observed by in vitro viral detection.
There are two generic subgroups of the RSV: A and B. Within these subgroups there is great variability and a general antigenic reaction of approximately 25%, mostly caused by G protein, which shows only 1% to 7% of antigenic activity. F protein is much less variable, with approximately 50% of antigenic reaction. During outbreaks, both subtypes are generally present; however, it is still debatable if the subtype A is related to more serious disease, and therefore it may be the cause of an increasing number of cases admitted into the intensive care unit.
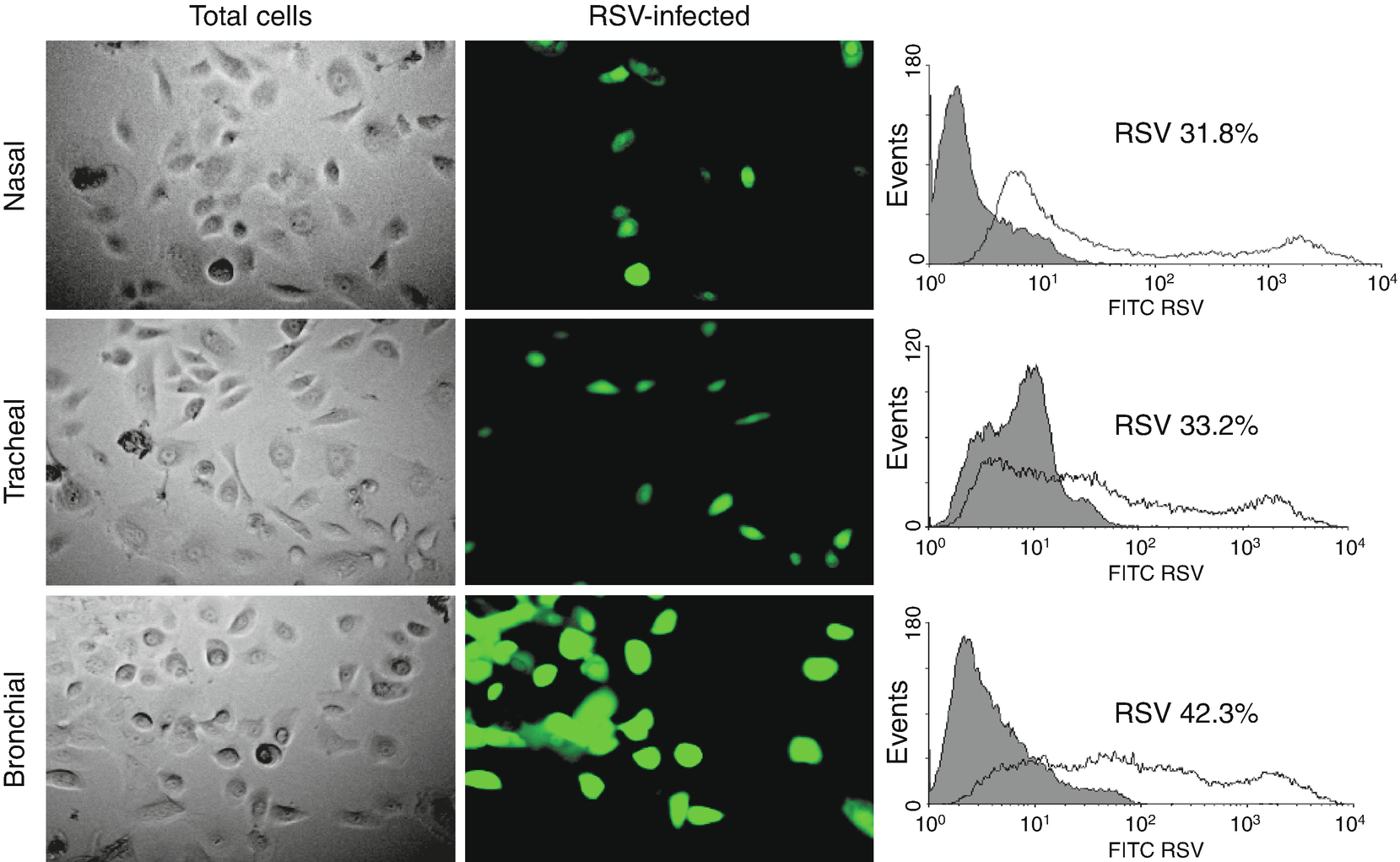
Epithelial cell infection in the respiratory tract. (a) Cells from human nose, trachea, and bronchial epithelial cells after being infected with RSV GFP expressing (rgRSV) to 1 MOI (multiplicity of infection) for 48 h. The clear field panels (left) show the total number of cells. The green fluorescence color (center) represents those actively infected with RSV. (b) Bronchial epithelial cells are more susceptible to RSV infection. Flux cytometry data show the percentage of fluorescent cells (infected) on each panel in comparison to control cells (not infected) (shaded histogram). Data are expressed as median ± SEM (n = 4 experiments). ∗∗∗P < 0.001 when compared to nasal or tracheal cells
Historically, it has been considered that the seriousness of the disease caused by RSV is related to the intensity of the host immune response. This perception comes from the postmortem examination of two children who died because of a pneumonia caused by this virus during the study of the formol-inactivated vaccine. These findings suggest that immunopathological mechanisms have an important role in the serious presentation of the disease caused by RSV. Also, studies done in animal models have indicated an amplified immunity for Th2 and activated cytotoxic cells, which would be responsible for this response, although several aspects of the physiopathology are still very controversial. Formol inactivation has been involved in what seems to be the cause of the immunogenicity amplification of the specific viral antigen, especially for the G protein of RSV. Nevertheless, the inactivation also caused an alteration in the capacity to induce a protective response of the neutralizing antibodies.
The infection caused by the RSV induces both humoral and cellular immune activity, and although this response does not achieve a complete response in relation to reinfection, it does seem to reduce the seriousness of subsequent infections. In infants, high titers of neutralizing antibodies against RSV, which come from the mother and are located in the umbilical cord serum, are related to much less risk of hospitalization because of bronchiolitis caused by this virus. This protection can also be obtained by the exogenous administration of specific immunoglobulin. Also, reduction of titers against RSV in the serum has been linked to a significant increase in the risk of developing a symptomatic infection. Cellular immunity probably has a function in the control of the active infection and the elimination of the virus. This idea is highlighted by the observation that immunocompromised subjects, whose cell-mediated immunity is reduced, tend to suffer a much more serious and extended RSV infection; also, they eliminate the virus for longer periods of time.
A recent study offers an alternate explanation of the physiopathology of serious diseases caused by this virus. This study examined the autopsy samples of 20 Chilean children who died of pneumonia with no intervention with mechanical respiration assistance. The authors compared the results with those of other infants who died of an infection caused by influenza, and there was no evidence of an inflammatory response in the most serious stage of the disease. There was no activation of cytotoxic T cells, but there was significant apoptosis induced by epithelial detachment. The authors interpret that the serious infection caused by RSV is the result of an insufficient adaptive immunity, and the elimination of the virus still depends greatly on a less efficient innate immunity.
Some recent studies have shown that RSV infection during the early stages of life promotes a great increase in nerve growth factor (NGF) and its receptors during the development of the respiratory tract, proteins that control the structural development of peripheral neurons, both afferent and efferent, and these cause changes in their functional activity in several ways that collectively define their “neural plasticity.” NGF overexpression induced by RSV may cause changes in both the short and long term related to the distribution and reactivity of sensorial and motor nerves through the respiratory tract. This change causes the unspecific hyperreactivity of the airway before and after the infection, which can be increased by chronic exposure to environmental pollution.
Studies in animal models indicate that overexpression in the NGF is crucial for the production of mucosal edema, mediated by neurogenic response, as well as innate lymphocytic and monocytic responses in the respiratory tract, when they have been infected by the RSV. This finding suggests that neuroimmune interactions created by neurotrophic pathways are very important in the systemic and local inflammation against viral pathogens. This important inflammatory mechanism is to a great extent resistant to steroids, which would give a plausible explanation for the poor therapeutic activity of these drugs in children with wheezing induced by this virus.
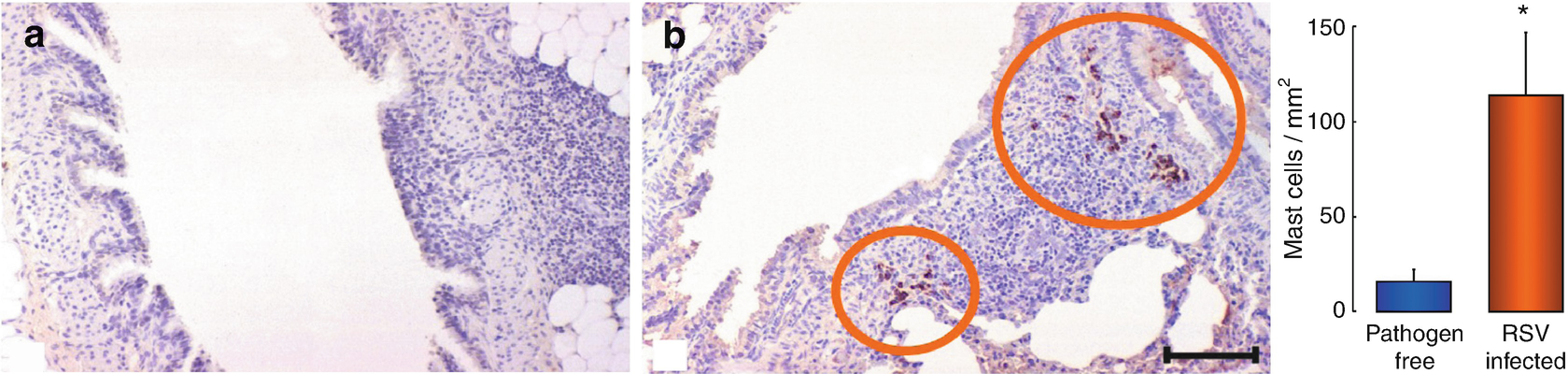
Mastocytes in pulmonary tissue. Lung sections of weanling rats, killed 5 days after being intranasally inoculated with virus-free environment (a) or suspension (b). Mastocytes have been identified through immunohistochemistry, using a tryptase-specific monoclonal antibody. A sevenfold average increase in mastocyte density was found in the lung sections of rats infected with respiratory syncytial virus (RSV), in comparison to those who were free of the pathogen (right). Inner scale = 40 m. ∗P < 0.05 significantly different to the rats who were free of the pathogen
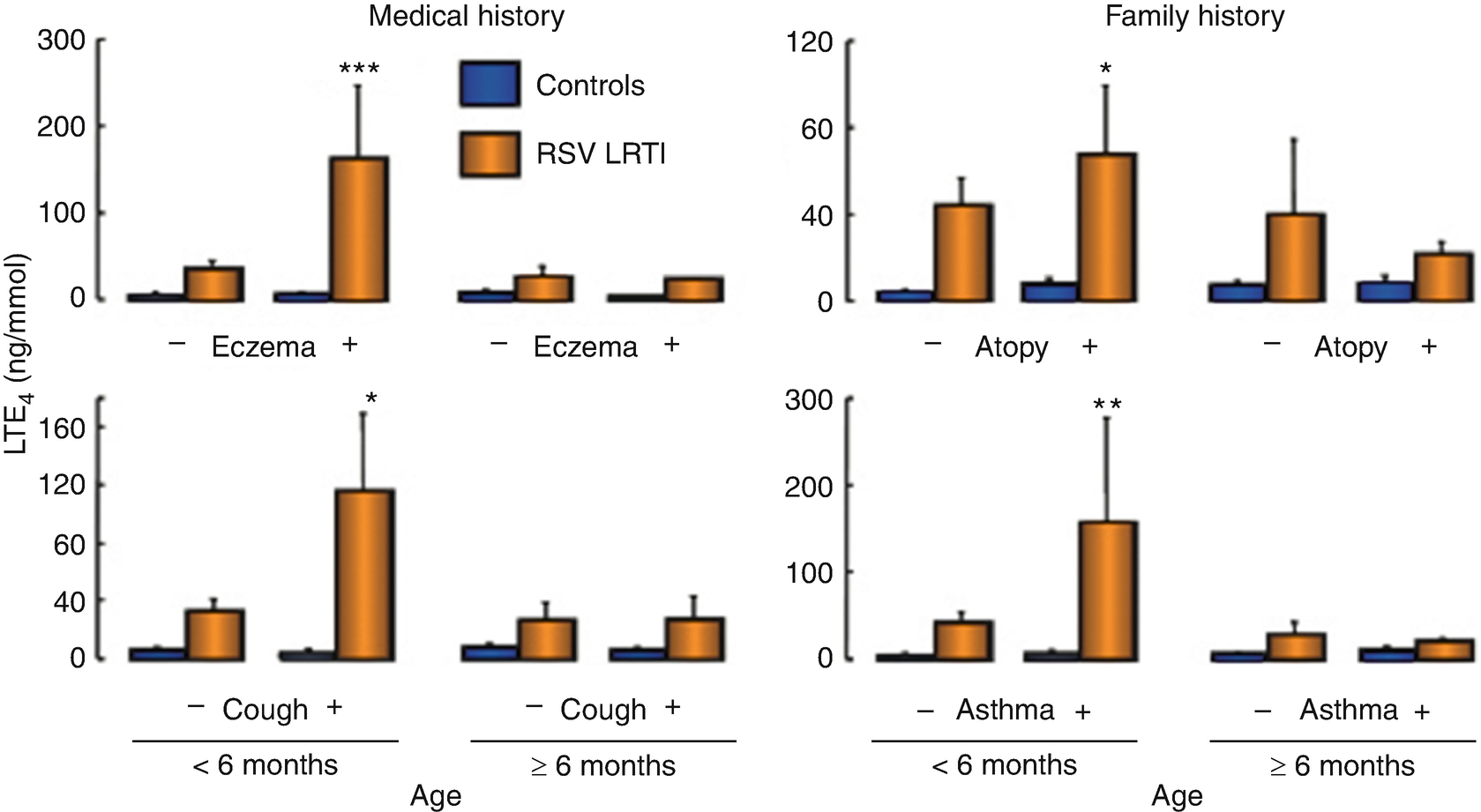
Leukotrienes synthesis. Urinary excretion of LTE4 (terminal product of CysLT metabolism) is increased in children with bronchiolitis caused by RSV when compared to control samples with no respiratory infection. Overproduction of CysLT shown by this marker is accentuated in younger patients (<6 months old) infected with this virus. Further, the effect of the virus seems to be amplified in infants with a higher concentration of LTE4, clinical history of eczema or dry coughing, and/or a familial history of asthma, an intrinsic predisposition to develop atopy, or hyperreactivity of the respiratory tract. Age or atopy alone does not seem to affect leukotriene synthesis when there is no infection. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001 significantly different from the control group of the same age free from respiratory infection
Analysis of infected pulmonary tissue has shown that the RSV effect in the leukotriene synthesis is temporary. The maximum levels are present during days 3 to 5 after the beginning of the infection and then return to baseline state after 30 days. These findings collectively suggest that the acute inflammatory response of the respiratory airways infected by RSV during the first years of life involves releasing CysLT, as well as the activation of the CysLT receptor. This effect can be observed in the antiinflammatory effect found in animal and human models in relationship to the leukotriene receptor antagonists, such as Montelukast. After the early phase of viral respiratory infection, leukotriene production and release quickly return to basal levels, but they can be reactivated when there are irritants present in the air (such as tobacco smoke), which can once again stimulate the nerve nociceptive fibers connected to the numerous mastocytes, which are still present in the pulmonary tissue.
Clinical Manifestations
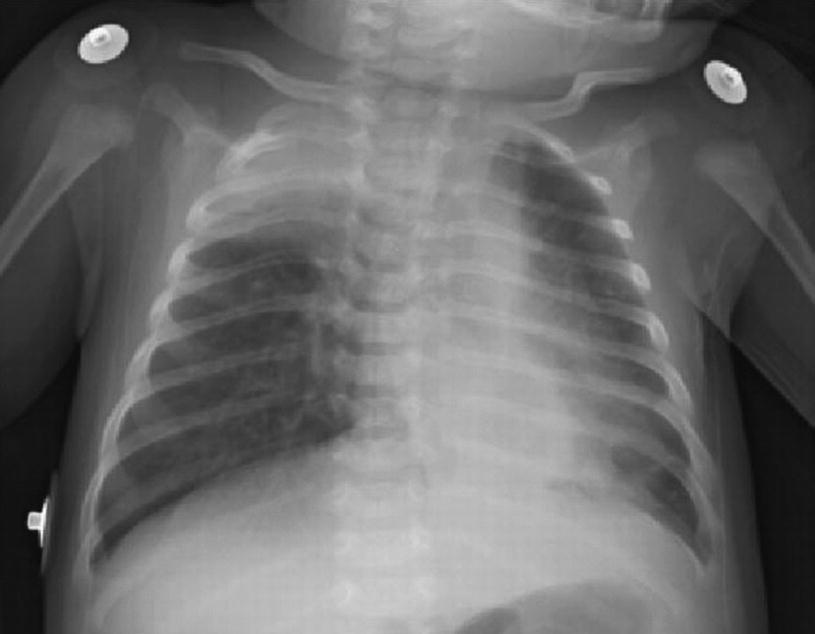
Acute bronchiolitis. Chest X-ray obtained from a child with bronchiolitis caused by RSV shows bilateral hyperinflation, irregular atelectasis, and peribronchial enlargement. Seriously ill patients may also present symptoms consistent with pneumonia, with interstitial infiltration areas
Patients undergoing serious involvement of the lower respiratory tract can also show changes on chest X-ray images that are more consistent with pneumonia, presenting areas of interstitial infiltration. The resulting respiratory difficulty may differ greatly in seriousness, ranging from minimum to extensive, and it can even be fatal. Infants tend to be lethargic, lack appetite, and present with otitis media. Elderly patients, as well as patients with cardiorespiratory disease or immunodeficiency, are also at risk to suffer a serious infection of the lower respiratory tract. Older children and healthy adults usually manifest symptoms compromising the upper respiratory airway, but they may as well present with a tracheobronchitis.
Apnea is a well-known complication of RSV in small infants and can be sufficiently serious to cause death. It affects about 20% of babies younger than 6 months of age who have undergone hospitalization. It is frequently the first clinical manifestation of virus infection, and its presence is not related to the seriousness of the disease or other related symptoms. Because of this, it is believed that the virus may be involved in at least some cases of infant sudden death, which also shares several epidemiological similarities with RSV infection, such as seasonal impact and risk factors.
Some studies suggest that infection by RSV significantly prolongs the duration of central apnea caused by a peripheral sensorineural stimulation reflex. The specific blockage of the central GABA-A receptors or the P (NK1) substance, as well as their high-affinity receptors, suppresses the influence originated by the RSV infection in relation to the apnea caused by sensorineural stimulation. This finding suggests that the P substance, released by the primary sensorineural neurons in the nodose ganglion, activates second-order GABAergic interneurons in the dorsal horn at the medulla. This process inhibits the function of the medullary inspiratory neurons, which causes the apnea.
Treatment
Most children infected by RSV have a mild disease, which is self-limited, and may only require symptomatic treatment that consists of a close follow-up, especially related to the progression of respiratory difficulty, oxygen need, and hydration. Children who refuse to be fed, who present with respiratory distress or need of supplemental oxygen, must be admitted into the hospital for closer observation and more aggressive management. Independently of where the patient is being treated, the base of the treatment is always to provide support care: this includes respiratory assistance and an adequate management of liquids and nutrition.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


