COMPLICATIONS OF
TRANSSEPTAL CATHETERIZATION
MEHUL B. PATEL, KHYATI PANDYA,
ATUL KHASNIS, RANJAN THAKUR
If you don’t know where you are going,
you will wind up somewhere else.
—Yogi Berra
In the early days of cardiac catheterization, the diagnosis of valvular and congenital heart disease required measurement of pressures in the left heart chambers. The initial approaches to obtaining left heart pressure measurement included the right paravertebral technique described by Bjork1 and the left bronchial puncture technique by Allison in 1953,2 followed by the direct left ventricular puncture procedure by Ponsdomenech and Brock in 1956.3,4 Transseptal puncture (TSP) for left heart catheterization (LHC) was first introduced by Cope and Ross in 19595,6 and later modified by Brockenbrough and Braun-wald in 1960.7 Mullins further modified this technique using a long sheath and dilator in 1983, and since then there has been very little change.8
Amongst transbronchial, transthoracic, and transventricular techniques for the assessment of left heart pressures, TSP has emerged as the most practical method.9 TSP also allows therapeutic intervention, which is not possible using the other methods. As the decades have elapsed, TSP has emerged as one of the most significant procedural developments in the field of interventional cardiology. In the late 1960s, however, this technique was associated with an aura of “danger and intrigue,” leading to a worldwide decline in the number of procedures. The advent of percutaneous transvenous mitral commissurotomy (PTMC) by Kanji Inoue, MD, in 1984 required transseptal catheterization (TSC) to reach the left atrium via TSP10 With this development, TSP reclaimed its importance and became the pivotal component of access to the left side of the heart for complex structural interventions.11 TSP became important to the electrophysiologist for ablation of left-sided accessory pathways and left atrial tachycardias starting in the early 1990s. However, it wasn’t until Haïssaguerre showed that atrial fibrillation (AF) is often caused by a rapidly firing focus in one of the pulmonary veins, thus ushering in the present era of AF ablation, that the TSP technique saw a worldwide resurgence.12 Commonly used indications for transseptal catheterization are listed in Table 15.1. Although safe in experienced hands, TSP can be a challenging and an unforgiving procedure. As there are very few large studies on TSP,13 most of the reported complications are from anecdotal case reports. In one series, the reported complication rate from TSC was 0.74% to 0.79%.14 This chapter describes many of the potential complications associated with TSP and offers important tips for anticipating, preventing, assessing, and managing those complications.
Anatomy: The Road map
Fossa Ovalis
The right and left atria are not lateral to each other; the right atrium (RA) is located anterolateral to the left atrium (LA). The LA is the most posterior cardiac chamber and forms two-thirds of the base of the heart. The interatrial septum (IAS) is therefore not a vertical midline structure, but rather slanted in the posterior-anterior plane from the right to the left, at a 65° angle (Figure 15.1). Embryo-logically, the IAS is formed by overlap of the septum primum and secundum; the gap in between, called the foramen ovale, is represented by the fossa oval is component of the IAS in adulthood.
The fossa ovalis comprises about 28% of the total interatrial septal area, which measures about 240 mm2 in adults.15 Though usually located posteriorly at the junction of the mid and lower third of the RA, its position can vary in hearts where the anatomy has been distorted by concomitant valvular or congenital heart disease. The muscular border of the fossa ovalis is 2-layered and formed by the folding of both the atrial walls. The anatomy of the inferior rim of the fossa ovalis also deserves attention. Anteroinferior to the fossa ovalis is the opening of the coronary sinus into the right atrium. The posterior aspect of the atrioventricular (AV) groove separating the LA from the left ventricle contains fibro-adipose tissue, and the AV nodal artery supplying the AV node (which lies in the triangle of Koch) runs through this section of the posteroinferior IAS.16 Excessive manipulation of the transseptal needle in this anatomical region can result in compromised AV nodal circulation. The artery to the sinus node (SN) originates from the right coronary artery in 55% cases and runs in the area around the superior vena cava (SVC)-RA junction.17 Though the RA is usually accessed through the inferior vena cava (IVC), in cases where the SVC is the route of entry, the anatomical location of the artery to the SN has important implications
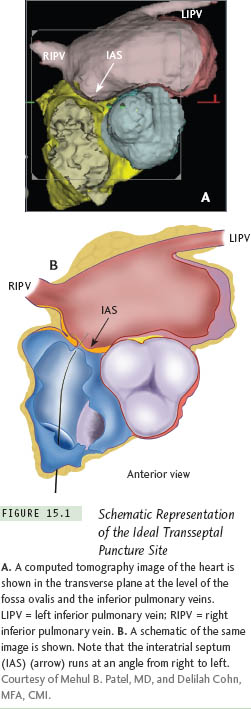
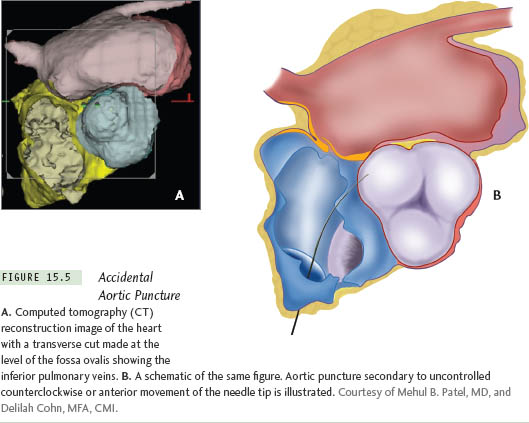
The LA has 3 components: a smooth-walled venous chamber, an atrial appendage lined by pectinate muscles, and a vestibule supporting the attachments of the leafets of the mitral valve. The pulmonary veins (PVs) enter the LA at the 4 “corners” of the venous chamber, enclosing a prominent atrial dome. The anterosuperior aspect of the fossa ovalis is related to the aortic root, and inadvertent puncture of this part of the IAS can result in a catastrophic aortic entry.
Atrial Septal Neighborhood
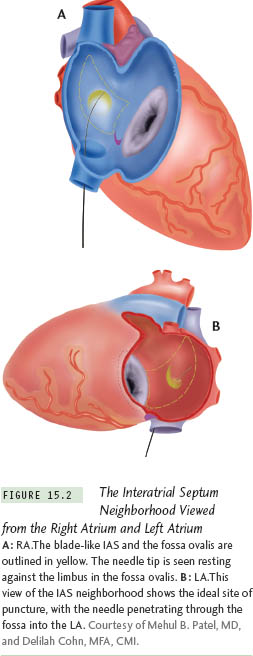
The IAS is a blade-like structure with a concave anterior curve formed by the ascending aorta and a convex posterior border. The posterior border is formed by the narrow expanse of the right atrial wall on the right and by the right pulmonary veins on the left. The tip of the blade is formed by the SVC on the right and by the right superior pulmonary vein on the left. The inferior margin is formed by the right atrial endocardium overlying the membranous interventricular septum (IVS) on the right and the mitral annulus on the left18–20 (Figure 15.2).
TSP Procedure
Originally, TSC was performed with only fuoroscopic guidance. As echocardiography came into use, transesophageal echocardiography (TEE) and intracardiac echocardiography (ICE) were integrated to improve safety and success rate. We describe some landmarks that can be useful for the fluoroscopy-guided procedure.
Though biplane fuoroscopy is preferable for TSP, single-plane fuoroscopy is usually suffcient. The instruments used for the procedure include a Brockenbrough needle, a 70-cm 14 F tapered tip polyethylene dilator, and a 7 F or 8 F transseptal sheath. A 5-mL or 10-mL syringe containing pure contrast medium is connected to the manifold, which is attached to the hub of the transseptal needle for constant pressure monitoring and also for contrast injection when needed. The anatomic and fluoroscopic landmarks, along with the “septal flush” or the “septal stain” method, can identify the IAS and fossa ovalis.
To identify the ideal site for TSP, the following steps can be helpful.
Steps and Landmarks
for Optimal TSP
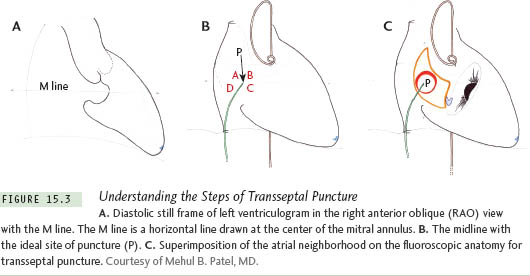
1. After obtaining a diastolic still frame of the left ventriculogram in the 30° right anterior oblique (RAO) view, draw a horizontal line from the center of the mitral valve (M line). (Figure 15.3a)
2. Take a cineangiogram with the pigtail sitting on the aortic valve in the frontal view. Draw a horizontal line from the lowest point of the pigtail horizontally to the right outmost border of the LA. Draw a perpendicular line from the midpoint of this line. (A horizontal line drawn from the summit of the His-bundle catheter recording a His-bundle electrocardiogram would also serve as a similar reference for the M line.) (Figure 15.3b)
3. The point of intersection of this perpendicular midline to the horizontal M line is the ideal site of puncture (P). It is assumed that the vertical midline divides the IAS into anterior and posterior halves (“midline”) (Figure 15.3c).
Contraindications to TSP
Few conditions constitute absolute contraindications for TSC.
LA Thrombus or Mass
Patients with mobile thrombi in the LA are at a high risk of systemic embolism, and TSP should be deferred in such patients. Patients with non-mobile thrombi in the LA cavity should be treated with long-term anticoagulation therapy to maintain an international normalized ratio (INR) between 2 to 3, and resolution of the thrombus should be ascertained before proceeding with LA cannulation and catheter manipulation. Presence of thrombi confined to the LA appendage (without protrusion into the LA cavity) is not a contraindication for PTMC.21–24 However, catheter ablation for AF should be deferred until patient has been anticoagulated for an adequate length of time and resolution of thrombus is documented.
Bleeding Diathesis or
Conditions Necessitating
Anticoagulation
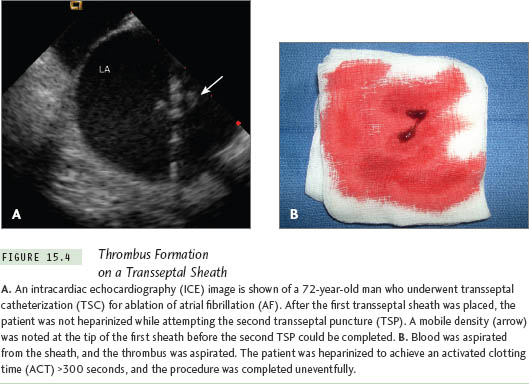
Patients undergoing TSC should be anticoagulated before TSP is done or immediately after the LA is entered. Within minutes of entering the LA, a thrombus may form in and around the sheath and lead to an embolic event, as shown in Figure 15.4. Thus, inability to anticoagulate a patient because of a bleeding diathesis or another contraindication, such as intracranial bleeding or gastrointestinal bleeding, constitutes an absolute contraindication for TSC.
Complications of TSC:
What the Mind Does Not Know the Eye Does Not See
TSC is generally a safe procedure in experienced hands. Most complications have been reported from the days of fluoroscopy-guided procedures. It may be reasonable to conclude that availability of ICE and TEE will reduce the risk of complications, as evidenced by the example illustrated in Figure 15.4.
An arbitrary classifcation of potential complications is provided in Table 15.2.
I. Related to TSP
A. Puncture-Related
1. Puncture of Sites Other Than IAS
This is a major complication of TSP, if not recognized early. Having a high index of suspicion for cardiac perforation is crucial for preventing catastrophic outcomes. Cardiac perforation may result in chest pain and may be accompanied by a decline in the arterial pressure, an increase in cardiac size on fluoroscopy, and loss of cardiac motion on fluoroscopy. Uncomplicated needle or catheter perforation is usually due to RA puncture. Such punctures cause chest pain, generally without any major sequelae.
a. Perforation of Aorta
Diagnosis Needle puncture of the aortic wall may lead to pericardial effusion. Low magnification fluoroscopy in left anterior oblique (LAO) view may show loss of cardiac pulsations at the costophrenic angle with or without an appreciable increase in the cardiac size.
Management Inform the surgical team and withdraw the needle. Watch for changes in heart rate and blood pressure for a substantial period of time on the catheterization table. Needle-only perforations usually seal off without need for surgery. In the absence of hemodynamic changes, a decision should be made whether the procedure should be continued or abandoned because the patient will require full intraprocedural anticoagulation. If the risk is unknown or deemed high, it may be best to abort the procedure. All such patients should be watched and monitored carefully for several hours in the intensive care unit. In case of hemodynamic compromise with effusion on echocardiography, the patient should be emergently wheeled to the operating room. If it is determined that the aorta is definitely punctured with the dilator and/or the transseptal sheath, it is best to leave the instrument in place to seal the entry site rather than withdrawing it and risking hemodynamic collapse.
Prevention It is a good practice to check the aortic root size before TSP. Have a high index of suspicion if the aortic root is dilated. Avoiding punctures medial to the midline (zones B and C of Figure 15.3b) could potentially prevent most inadvertent aortic punctures. Also, it is vital to feel the transmitted pulsations with the right-hand fingertips holding the needle hub. Importantly, it should be remembered that the aorta can be punctured even after successful entry into the LA if the needle is pointed anteriorly. Hence, continuous needle pressure monitoring is crucial. Recording a left ventricular (LV) pressure waveform could mean that the needle has punctured the aorta and entered the left ventricle retrogradely. (See Figures 15.5 and 15.6.)
b. Perforation of RA, LA, or LV
Diagnosis of Tamponade Asymptomatic pericardial effusion not requiring intervention is reported in 2.5% of cases.25 Pericardial tamponade requiring intervention is reported in 1% of all cases undergoing TSP.25 Loss of cardiac borders on fluoroscopy with loss of cardiac pulsations generally precedes the hemodynamic signs; one should never wait for the classical Beck’s triad of hemodynamic collapse.26 Acute cardiac tamponade due to perforation during cardiac intervention is a potentially lethal complication with a mortality rate as high as 42%.27 Hypotension is usually the first hemodynamic sign. Bradycardia is another important early sign of perforation. These hemodynamic clues should alert the operator to the possibility of tamponade, but these changes are not very specifc and may be related to a variety of other causes, such as sedation and alteration of autonomic tone. Furthermore, it is extremely crucial to realize that tamponade can occur even in the absence of elevated venous pressures. Blood accumulates first in the posterolateral pericardial sac as the pericardium is attached anteriorly to the sternum and posteriorly to the spine. This region is best visualized as the lateral heart border in the LAO view. An emergency transthoracic echocardiogram (TTE) is also crucial to diagnose a global or a localized effusion. In rare cases, there may be serious significant bleeding into the posterior pericardial space, leading to the missed diagnosis of tamponade with a “dry” tap on pericardiocentesis.
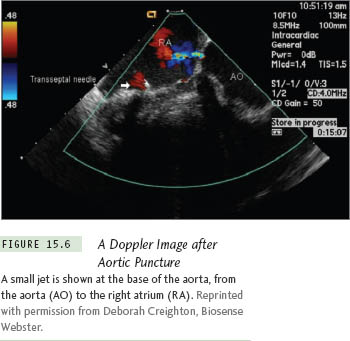
A small jet is shown at the base of the aorta, from the aorta (AO) to the right atrium (RA). Reprinted with permission from Deborah Creighton, Biosense Webster.
Uncontrolled posterosuperior movement of the needle can cause puncture of the right atrial and left atrial walls in the transverse sinus, permitting needle entry from the RA into the LA cavity. This “phenomenon of stitching” can be catastrophic if the passage is subsequently dilated with the dilator and the sheath. As long as the sheath is in place, the patient may remain asymptomatic. However, once the sheath is removed, rapid hemodynamic collapse may ensue because large rents are created in both the RA and LA (Figure 15.7; see also Figure 15.8).
Further posterosuperior movement of the needle can cause puncture of the RA in the transverse sinus (Figure 15.9).
Steady advancement of the needle after transseptal puncture can cause puncture of the LA roof or the left superior pulmonary vein (Figure 15.10).
For procedures such as PTMC, LV perforation is a potential complication. Left ventricular hypertrophy does not attenuate the risk of LV perforation. In fact, perforation of a hypertrophied ventricle appears to occur just as readily as a normal ventricle.
Joseph et al investigated mechanisms underlying 10 cases of cardiac perforation with tamponade among 903 balloon mitral valvuloplasty procedures. Two mechanisms of hemopericardium related to TSC were proposed: (1) perforation of the aortic root and adjacent RA by upward displacement of the transseptal apparatus, (2) tear of the posterior RA wall by dilatation of the track produced by very low IAS punctures. Operator experience (inversely related) and patient age (directly related) were predictors of cardiac perforation in this study28
Management of Tamponade
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


