NOVEL APPROACHES AND TECHNOLOGY
FOR TRANSSEPTAL CATHETERIZATION
SHELDON M. SINGH, VIVEK Y. REDDY
Transseptal catheterization was first described by Ross in 19591 to provide access to the left atrium (LA) during diagnostic left heart catheterization. Although initially popular, the development of pulmonary artery flotation catheters and of the retrograde aortic approach to accessing the left ventricle resulted in a diminished need for transseptal catheterization. However, there is now a resurgence of interest in this procedure due to the increasing numbers of “left-sided” electrophysiological and interventional cardiac procedures, including catheter ablation of cardiac arrhythmias such as atrial fibrillation, implantation of left atrial appendage occlusion devices, percutaneous cardiac valvular procedures, and placement of percutaneous ventricular assist devices. Despite a high success rate (>99%) and a low (0.8%) complication rate2 in experienced hands, variations in the position and structure of the interatrial septum may result in challenging punctures and increase the risk of inadvertent perforation of adjacent structures, such as the aortic root, the coronary sinus, and the posterior right atria wall. Although the current technique for transseptal access has changed little since first described, tools are being developed to improve the ease and safety of this procedure. This chapter will review these novel approaches with specific attention to innovative methods of visualizing and perforating the fossa ovalis.
Current Approach to Transseptal Catheterization
The transseptal apparatus is withdrawn inferiorly, watching for the first dilator jump, which occurs as the system falls off the torus aorticus, followed by a second jump as the system falls off the limbus of the fossa ova-lis. At this point, the transseptal apparatus is then advanced slightly (1-2 mm) to ensure it is in contact with the fossa ovalis. To stain the fossa ovalis and demonstrate tenting, 3 cc to 5 cc of contrast may be injected. Once anti-coagulation is administered (150 U/kg heparin bolus), the needle is advanced across the interatrial septum and into the LA. Crossing of the interatrial septum may be confirmed with contrast injection into the LA, analysis of blood oxygen saturation, or with pressure monitoring. A 0.014-inch angioplasty wire may be inserted through the lumen of the Brockenbrough needle into a pulmonary vein; this technique may both confirm the location of the needle in the LA and theoretically minimize inadvertent movement and puncturing of adjacent structures by the transseptal system. On the other hand, because of the extreme thrombogenicity of metal, it is advisable to minimize the time the wire is in contact with the blood pool.
Once the needle has crossed the interatrial septum, the transseptal system is rotated anteriorly (ie, counterclockwise to approximately 3 o’clock) to avoid puncturing the posterior LA. The dilator is advanced over the needle, followed by the sheath over the dilator; a stained fossa ovalis allows the operator to gauge the depth of the sheath within the LA. The sheath is then stabilized, and the dilator and needle are removed as a unit.
Many electrophysiology laboratories perform transseptal punctures solely with the aid of fluoroscopy. A pigtail is often placed in the aortic root to mark the level of the aortic valve. Alternatively, a catheter may be placed at the level of the His bundle to approximate the inferior aspect of the aortic valve. The posterior and lateral borders of the atria are identified on the RAO and LAO views, respectively. Generally speaking, the fossa ovalis lies approximately 1 cm to 3 cm inferior to the level of the aortic valve. In the RAO view, the fossa ovalis is en face. Thus, this view is useful to tailor the anterior-posterior septal location of the puncture according to the clinical need; for example, for catheter ablation of atrial fibrillation, a posterior puncture is preferred, but during left atrial appendage occlusion, a more anterior septal puncture is preferred. Although fluoroscopy provides excellent spatial resolution to allow real-time visualization of the needle and sheath assembly, it is limited in its ability to resolve soft tissue. In addition, traditional fluoroscopic landmarks may not hold for all groups of patients; for example, the successful transseptal puncture position has been shown to be fluoroscopically posterior and higher in older individuals.3
Methods to Visualize the Fossa Ovalis During Transseptal Catheterization
The inability to identify the fossa ovalis may be responsible for approximately half of all failures to enter the left atrium during transseptal catheterization.4 Imaging techniques including the use of echocardiography, magnetic resonance imaging (MRI), computed tomography (CT), electroanatomic mapping and direct endoscopic visualization of the fossa ovalis can complement traditional fluoroscopic approaches to identifying the fossa ovalis.
Echocardiography
The use of echocardiography to guide transseptal catheterization has been extensively studied.5–8 While transthoracic echocardiography (TTE) may permit visualization of the interatrial septum and adjacent structures, its role in guiding transseptal catheterization is limited due to poor image quality, disruption of the sterile field, and the requirement of a second operator. Concomitant transesophageal echocardiography (TEE) can be performed with the use of fluoroscopy without disruption of the sterile field; however, this technique also requires a separate operator. In many centers, intracardiac echocardiography (ICE) is routinely used, given its ability to delineate the anatomy of the interatrial septum and adjacent structures without the need for a separate operator.
Intracardiac Echocardiography
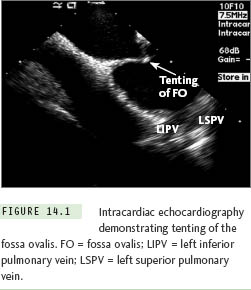
Currently 2 types of ICE catheters are available for use: (1) 64-element phased array ultrasound systems, which allow imaging of a sector in the plane of the ICE catheter (either ACUSON AcuNav [Siemens Healthcare, Mountain View, CA] or ViewFlex [EP MedSystems, Inc, West Berlin, NJ]), and (2) an ultrasound transducer with a single rotating crystal that provides a circumferential field of view (Ultra ICE [Boston Scientific, Inc, Natick, MA]). Via femoral venous access, the ICE catheter is placed in the mid-right atrium, and the fossa ovalis is identified. The transseptal system may be identified when it is positioned in the area of the fossa ovalis. With gentle advancement of the transseptal system, tenting of the fossa may be demonstrated with ICE (Figure 14.1). Once the interatrial septum is punctured, contrast or saline is injected through the transseptal needle and is visualized in the LA with ICE. For atrial fibrillation ablation procedures the fossa is punctured at a mid-low level with the left pulmonary veins in the ultrasound plane; this seems to provide the ideal access to the LA sites targeted during this ablation procedure.
In addition to identifying normal fossa anatomy, ICE can be quite helpful in identifying anatomic variants, including septal thickening, septal aneurysms, and congenital anomalies, such as the presence of a double-layered fossa (Figure 14.2). Of note, care must be taken when interpreting the position of the transseptal apparatus with ICE, due to the reverberation artifact from the transseptal needle. Finally, ICE is extremely useful to assess for possible complications related to the transseptal procedure, such as (1) inadvertent posterior puncture resulting in pericardial effusion, (2) inadvertent puncture of the aorta, (3) inadvertent puncture of the posterior LA wall, resulting in an LA wall hematoma, or (4) formation of a thrombus related to transseptal apparatus or septal puncture site.9,10
Three-Dimensional Transesophageal Echocardiography
Three-dimensional (3-D) ultrasound is now available with transesophageal imaging probes, allowing for high-resolution, realtime volume imaging of cardiac structures. The greatest potential advantage that 3-D TEE has over traditional 2-D TEE is its easy-to-understand volumetric image of the fossa ovalis and transseptal apparatus (Figure 14.3).11,12 One limitation of 3-D TEE is the
somewhat increased time required to properly segment and present the image to the operator in a clinically useful and timely manner. However, with increased availability and experience, this imaging modality is likely to have a more widespread role in guiding transseptal catheterization.
Real-time MRI
Echocardiography is frequently limited by acoustic shadowing and the inability to obtain adequate imaging windows. Despite this, the strength of echocardiography lies in its ability to provide real-time visualization of the fossa ovalis and transseptal system during transseptal catheterization. Recently, Raval et al13 reported their experience with real-time MRI guidance of transseptal punctures in swine. Using custom designed MRI-compatible transseptal needles and sheaths, atrial septal punctures and balloon atrial septotomies were performed in a 1.5-tesla MRI interventional suite. Clear visualization of the fossa ovalis and transseptal apparatus was observed with MRI imaging. Atrial septal puncture was successful in all animals; however, catheter malfunction lead to hemorrhage in one animal. With the development of human-grade MRI compatible catheters, real-time MRI-guided transseptal puncture may be possible in clinical practice.
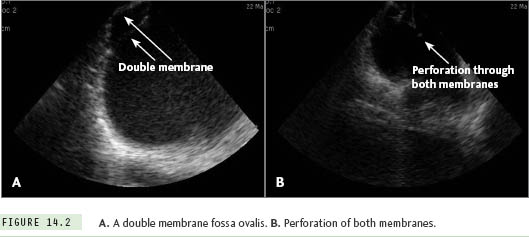
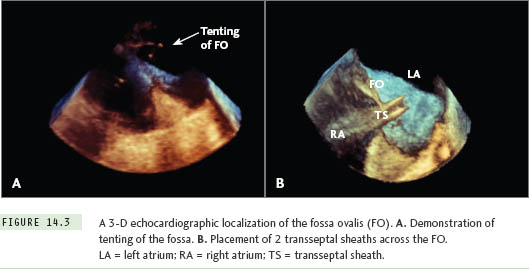
Image Integration
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


