TRANSCATHETER ATRIAL SEPTAL DEFECT DEVICE CLOSURE
SAWSAN M. AWAD, QI-LING CAO, ZIYAD M. HIJAZI
Transcatheter closure of secundum atrial septal defects (ASD) has become an accepted alternative to surgical repair. With more advances in device manufacturing and the availability of a broad array of devices, decreasing complications and higher closure rates have been reported. In this chapter, we will discuss the technique of percutaneous ASD device closure and the commonly encountered complications and problems during the procedure.
Equipment
Performing interventional cardiac catheterization for congenital heart diseases (CHD), both in adults and children, requires well-trained cardiologists and a well-equipped cardiac catheterization laboratory. Biplane fluoroscopy imaging is preferred, but not essential, for ASD device closure, as it allows the interventional cardiologist to have better assessment of the anatomy and location of the defect, and it minimizes the amount of contrast agent given during the procedure. All types of wires, catheters, balloons, retrieval catheters/snares, and devices (in an array of sizes) should be readily available in the catheterization laboratory that does this type of procedure. The use of the wrong-size device or a failed procedure or complication due to a lack of the proper equipment is unacceptable.
Atrial Septal Defect
Closure atrial septal defects (ASDs) represents a communication between the right and the left atria. Surgical repair of simple ASDs was among the first congenital cardiac abnormalities to be corrected. This became more common after the advent of cardio-pul-monary bypass. However, surgical correction entailed a small risk of morbidity and mortality. With the advent of closure devices that could be delivered via a catheter in the 1990s, non-surgical closure of these defects became possible.
Anatomical Defects
ASD accounts for approximately 19% of all CHD.1 The ostium secundum defect is the most common type, and accounts for 60% to 70% of all ASDs and for 30% to 40% of all CHD in patients older than 40 years. This is the type of ASD that is amenable for percutaneous device closure. Another type, the ostium primum defect, accounts for 15% to 20% of ASDs, and a third type, the sinus venosus defect, is seen in 5% to 15% of ASD patients. The latter 2 types are not amenable for percutaneous closure using the currently available devices/techniques.
Procedure Indications
Indications for closure of secundum ASD are related to patient symptoms and the risks of having atrial level shunting. Patients with ASDs are usually asymptomatic in the first 2 decades of life. We believe the most important criterion of shunt significance is the presence of right ventricle volume overload as shown by transthoracic echocardiography. If the Qp:Qs ratio (pulmonary to systemic flow ratio) is more than 1.5:1, such patients may experience symptoms of shortness of breath and fatigue.2 However, calculation of the Qp:Qs ratio is not required to judge the significance of the shunt and may have some flaws. The presence of left-to-right shunting across the defect will lead to the development of right ventricular volume overload and, later, right ventricular dysfunction, progressive pulmonary vascular disease, and atrial dysrhythmias. In addition, patients with an atrial communication may be at risk of development of paradoxical embolus, leading to stroke, transient ischemic attack, or peripheral emboli.2–4
Contraindications for Transcatheter Device Closure
Only secundum type defects with appropriate rims are eligible for device closure. The presence of at least a 5-mm rim of tissue around the defect is needed to be able to anchor the device. The superior, inferior, and posterior rims are essential; however, absence of the anterior rim is not required when using the Amplatzer device. Size of the defect by itself does not constitute an indication or contraindication for device closure. However, one needs to be careful placing large devices that occupy the entire septum. We have placed devices in children that occupied the entire septum; as these patients grow in size, the device becomes smaller for that septum (personal observation).
Other types of ASDs are not appropriate for the currently available devices. These include sinus venosus defects (of the superior and inferior type) and ostium primum defects. Other contraindications for closure include the presence of active infection (within 1 month of closure) and the inability to take antiplatelet therapy.
Available Devices
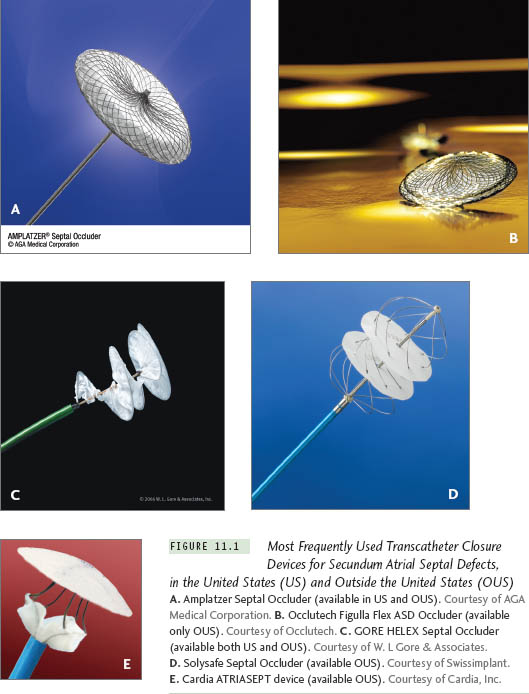
The first successful transcatheter closure of a secundum ASD was performed by King and Mills in the early 1970s.5 The devices currently approved by the U.S. Food and Drug Administration (FDA) for clinical use are the Amplatzer Septal Occluder (AGA Medical Corporation, Plymouth, MN), and the GORE HELEX Septal Occluder (W. L. Gore and Associates, Inc, Flagstaff, AZ).
Amplatzer Septal Occluder (ASO) devices provide good closure rates (97%) with comparable complication rates to open-heart surgery.6,7 The GORE HELEX Septal Occluder consists of an expanded polytetra-fluoroethylene patch material with hydro-philic coating, supported by a nickel-titanium (nitinol) super-elastic wire frame in the shape of a coil. This device is not suitable for defects larger than 18 mm as sized by a balloon catheter.8 Various modifications of the ASO have been reported, including the fenestrated ASO and the cribriform ASO devices for multi-fenestrated ASDs or ASDs with septal aneurysms.9,10 There are other devices available outside the United States for device closure of secundum ASDs. These include the Occlutech Figulla device and its new generation the Figulla Flex (Occlutech, Jena, Germany), the Cardia ATRIASEPT device (Cardia, Inc, Eagan, MN), and the Solysafe Septal Occluder (Swissimplant, Solothum, Switzerland). Figure 11.1 depicts some of the devices that are available both in and outside the United States.
Imaging During ASD Device Closure
In addition to fluoroscopic imaging, intracardiac echocardiography (ICE) is the tool preferred for guiding ASD closure in both adults and children11; however, due to the cost of the ICE catheters, many centers still use transesophageal echocardiography (TEE) to guide the closure procedure.
Operator Training
When embarking on a transcatheter closure of an ASD, it is imperative that the operator has very good experience and is versed in all aspects of interventional cardiology and that the laboratory is equipped with all types of catheters, snares, and delivery sheaths and has a surgical team in-house for bailout situations. When the devices were approved in the early 2000s, the manufacturer, along with the FDA, required operators to be trained (proc-tored) on the use of these devices. Training is supervised by the manufacturer and involves didactic lectures, simulation, and live cases. For more information on training requirements, the reader can visit the Web site of any of the manufacturers.
Closure Protocol
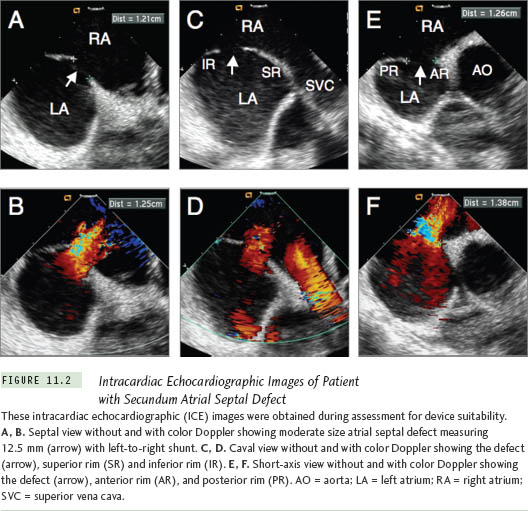
The procedure is performed under conscious sedation with the use of continuous ICE imaging for guidance. Vascular access is usually through the right femoral vein. For patients >35 kg in weight, the right femoral vein is accessed via 2 separate punctures, a few millimeters apart, 1 for the delivery system and 1 for the 8 F ICE catheter. Routine right and left heart catheterization is performed, followed by assessment of the degree of left-to-right shunt. Full assessment of the defect, surrounding rims, and remaining cardiac structures should be performed prior to device insertion (Figures 11.2 and 11.5). Heparin is administered routinely in all patients to achieve activated clotting time (ACT) >200 seconds at the time of device deployment. Angiography is performed in the right upper pulmonary vein in the hepa-toclavicular projection to assess the size and location of the defect. This step is an optional one. We usually do this in our laboratory, and use this angiogram as a road map when we deploy the device. Selection of the device size is performed according to the “stop flow” diameter of the defect (Figures 11.3a, 11.6a). This is performed under echocardiography with color Doppler. The balloon is inflated across the defect until there is cessation of shunt. Once there is cessation of shunt, a freeze-frame by echocardiography (Figures 11.3a, 11.6a) and cine-fluoroscopy (Figures 11.4b, 11.7b) is performed to measure the size of the balloon. For the ASO, a device no more than 2 mm larger than this diameter is chosen for closure, and for the GORE HELEX, a ratio of 1.7-2X this size is chosen. Balloon sizing in our center is optional. We usually use a device 25% to 30% larger than the 2-D diameter by ICE for the ASO device.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree