CHALLENGING TRANSSEPTAL
CATHETERIZATIONS
YU-FENG HU, MING-HSIUNG HSIEH,
SHIH-ANN CHEN
Although the transseptal puncture (TSP) technique has been shown to be safe in the vast majority of cases, this procedure can result in life-threatening complications, such as a cardiac tamponade, systemic emboli, or aortic perforation. The reported complication rate has ranged from 0.74% to 1.3%.1 Complications during TSP are more likely when the cardiac anatomy is unusual or distorted, as in cases of congenital heart disease with or without surgical correction. However, TSP has become important for delivering modern therapeutic options to patients with cardiovascular diseases. To further improve the success rate and to avoid any complications, several imaging techniques have been developed to clarify the anatomy and to guide the TSP, including atriography, transesophageal echocardiography (TEE), and intracardiac echocardiography (ICE). Several new methods have recently been proposed to overcome any difficulty during the transseptal puncture; these methods include the use of a radiofre-quency-assisted transseptal puncture or access through a patent foramen ovale (PFO).2
In this chapter we will describe some caveats regarding transseptal catheterization in patients with normal anatomy, discuss tips on TSP in patients with challenging anatomy, and, finally, provide guidelines for the use of ancillary imaging modalities to prevent complications in challenging cases.
Caveats for Transseptal Catheterization in the Normal Heart
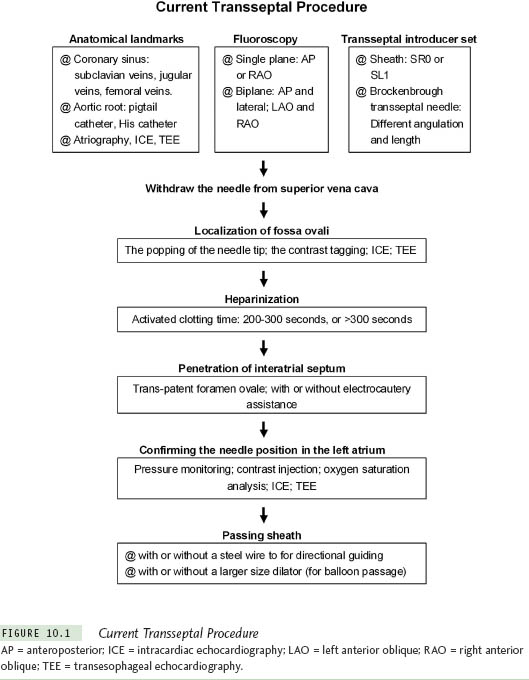
Different modalities and instruments are used to perform TSP. Knowing the different instruments and methods for TSP helps the operator to overcome difficulties by choosing the most effective approach. In Figure 10.1, various steps and tools for TSP are listed. The general principles for TSP are described as follows.
Before TSP, the anatomical landmarks need to be clarified. The inferior margin of the interatrial septum is delineated by the coronary sinus catheter. The anterior roof of the interatrial septum is the aortic root, which can be marked either by a catheter at the His bundle, advanced through the venous systems, or by a pigtail catheter in the aorta, inserted retrogradely. Some centers use the right atriography routinely to delineate the interatrial septum, right atrium, and associated anatomical details. The left atrial border, pulmonary vein, and sometimes the aortic root can be clarified in the venophase of the right atriography. The use of ICE and TEE can be very helpful in defining the anatomy of both the atria and the interatrial septum.
To guide TSP, most centers use fluoroscopy, which may be biplane or single-plane with different projections. A variety of pre-shaped sheaths, such as the SL1 and SRO, are commonly used for catheter ablation of atrial fibrillation. Each curvature of the sheath can facilitate manipulation in different regions of the left upper chamber. Brock-enbrough (BRK) transseptal needles have different lengths (71 cm for the adults and 56 cm for the pediatrics). Two different transseptal needles are commonly used: A BRK (small-curved, the angle between the distal curved portion and the shaft is ∼19°) and a BRK-1 (large-curved, the angle between the distal curved portion and the shaft is ∼53°). The small-curved needle is the best first choice in most patients. However, for older patients or patients with large right and/or left atria, the large-curved needle may be the best first choice. If required, the curve of the transseptal needle can be modified manually.
Confirmation of the location of the fossa ovalis is a critical step for TSP. The sudden medial drop of the needle tip moving from the superior vena cava (SVC) to the fossa ovalis and contrast staining of the septum are 2 common methods. ICE and TEE are very helpful in showing the contact between the transseptal needle and the interatrial septum as well as tenting of the septum.
Once transseptal catheterization is performed and the sheath is placed in the left atrium, a thrombus may form within a very short time (see “Chapter 12). To prevent this, patients are often heparinized prior to transseptal catheterization. This may seem counterintuitive given the risk of aortic puncture and cardiac perforation. Different intensities of heparinization are used. In our center, an activated clotting time (ACT) from 200 seconds to 300 seconds is considered appropriate. However, in some centers, an ACT higher than 300 seconds is used, while others give a bolus of 5000 units of heparin without measuring the ACT at this stage of the procedure.
The penetration of the needle through the interatrial septum is achieved by manual force. Electrocautery or radiofrequency energy may be used to facilitate this part of the procedure. It is very important to confirm that the needle is within the left atrium before advancing the dilator or the sheath. Recoding the left atrial pressure waveform or contrast injection are 2 common methods. Oxygen saturation analysis to show high oxygen content may be useful. For the passage of the dilator and the sheath, some operators simply advance the sheath and dilator after the penetration of the transseptal needle, while others prefer to pass a guidewire through the transseptal needle to guide the direction before the advancement of the sheath and dilator.3 For mitral valvuloplasty and balloon-based ablation devices, a large-size vascular dilator (up to 14 F) may be required for passage of the catheter.
Complex Congenital Heart Diseases
Although it is thought to be highly risky to perform TSP in patients with complex congenital heart diseases, Mullins and his colleagues reported a series of studies that demonstrated TSP is safe in these patients.4–6From February 1975 to October 1976, TSP was reported in 80 patients using the original Brockenbrough technique. Moreover, using the modified technique with a long sheath developed by Mullins, TSP was reported in 520 patients from July 1978 to June 1982, and in 217 patients from October 1983 to September 1998. The majority of patients in these 3 studies were infants and children, many with congenital heart disease. Cardiac defects in these patients included 158 with ventricular septal defects; 141 with aortic valve disease; 91 with tetralogy of Fallot, truncus arteriosus, pseudotruncus arteriosus, or postoperative tetralogy; 88 with coarctation of the aorta; 61 with a patent ductus arteriosus; 48 with multiple left heart obstructive lesions; 45 with transpositions including ventricular inversions; 48 with mitral valve disease; 23 with double outlet right ventricles; 28 with cardiomyopathies; 8 with hypertrophic subaortic stenosis; 6 with pulmonary stenosis; and 42 with miscellaneous abnormalities. In summary, a total of 787 procedures were associated with a complication rate of 4.3%: 33 pericardial punctures (2 with cardiac tamponade) and 1 ascending aorta puncture. There were 6 failed procedures. The cardiac defects of 4 of the patients in whom a failed TSP was reported included pseudotruncus arteriosus, aortic stenosis with aortic insufficiency, coarctation of the aorta and aortic stenosis, and gigantic right atrium. Two TSPs were abandoned due to pericardial puncture. The complication rate of the TSP in patients with complex congenital heart disease seems to be acceptable and only a little higher than that in the general population. On the other hand, operator experience is a major determinant of procedural success and safety, and the vast experience reported by Mullins et al is unlikely to be accrued in most laboratories today. Before the procedure, several issues that contribute to safe and successful TSP need to be addressed.
Steps for Avoiding Major Complications with an Unusual Anatomy
1. Operator skill and experience are of considerable importance for transseptal puncture in patients with an unusual anatomy.
2. Delineate bi-atrial anatomy with auxiliary images, such as atriography, intracardiac echocardiography, or transesophageal echocardiography.
3. Continuously monitor the intracardiac pressures throughout the procedure, or use a contrast tag to confirm the needle position if needed.
4. Never advance the needle until the catheter has been perfectly positioned on the fossa ovalis.
5. Never advance the catheter until the needle has clearly entered the left atrium, as confirmed by a distinct left atrial pressure recording, contrast injection, and a free position of the needle on fluoroscopy.
First, because the cardiac anatomy, especially of the interatrial septum, changes after cardiac operations, auxiliary images to clarify the atrial anatomy are necessary, such as right atriography, ICE, or TEE. Second, confirming the puncture site with contrast tagging (“staining” the septum), continuous pressure monitor, or auxiliary images (ICE, TEE) are important before penetrating the septum. At times, the direction of the needle may need to be modified, based on the altered anatomy of the interatrial septum. Third, sometimes, it is very difficult to penetrate prosthetic material. This may require electrocautery or other instruments. Finally, the needle position needs to be confirmed before advancing the sheath.
The Position of the Transseptal Needle in Complex Congenital Heart Disease
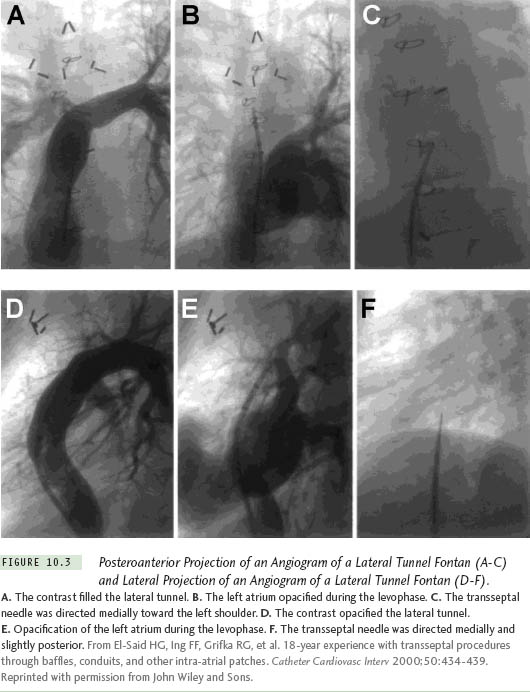
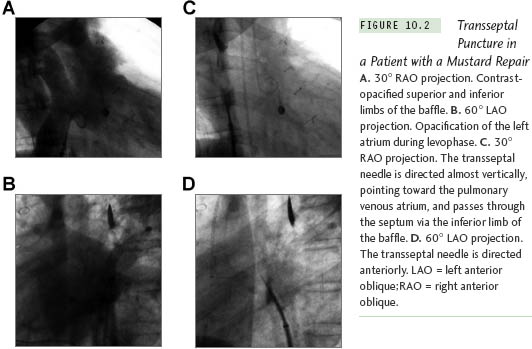
The position of the needle will differ because the cardiac anatomy changes after a cardiac operation. Figure 10.2 illustrates a patient after a Mustard procedure in our center who presented with typical atrial flutter. The TSP was performed successfully, as well as the catheter ablation of the atrial flutter. Right atriography was performed, using 30° right anterior oblique (RAO) and 60° left anterior oblique (LAO) views, which delineated the position of the interatrial septum. The transseptal set was withdrawn until the transseptal tip dropped off the native septum, onto the patch. The tip of the transseptal needle was directed anterior in the LAO view and aimed at the right shoulder in the RAO view. The transseptal needle successfully passed the inferior limb of the patch in the RAO view, requiring some force. In patients with a total anomalous pulmonary vein return or an atrial septal patch, using the anteroposterior and lateral projections, the needle is directed posteriorly and leftward toward the expected position of the left atrium. With Fontan baffles, the transseptal needle is oriented, as in patients with a normal heart, medially to the left and slightly posterior (Figure 10.3). Knowing the direction of the transseptal needle will help the operator perform the TSP more efficiently. Table 10.1 lists the changes in the needle position relative to anatomical variations, which helps to localize the needle when a TSP is performed under fluoroscopy.
Transseptal Puncture Through Prosthetic Material
TSP in postoperative patients with complex congenital heart disease has been considered more challenging. However, according to previous reports, it can be performed safely without any sequelae. Guided by fluoroscopy, El-Said et al7 reported 39 patients (1-31 years old) with intraatrial patches, including 16 with a D-transposition of the great arteries after a Mustard or Senning procedure, 4 with single ventricle variants post-Fontan operation, 4 with total anomalous pulmonary venous return repairs, 9 with atrioventricular canal repairs, and 6 with atrial septal defects with patch repairs. The material for the repairs included pericardium, native atrial tissue repair, Dacron, GORE-TEX, and Teflon. Only 1 transseptal puncture in a 17-month-old child with a status post-Senning repair failed because the intraatrial patch could not be traversed. Echocardiographic fol-low-up was available in 30 patients, and no residual shunts were noted in 28 patients. In 2 patients, the shunts were created intentionally (a fenestration). No complications were reported. Similarly, Perry et al reported 2 successful TSP procedures in 2 patients with a D-transposition of the great arteries, using a fluoroscopy-guided transseptal approach.8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree