Tuberculosis
INTRODUCTION
Tuberculosis (TB) is a severe and contagious disease caused by infection with members of the Mycobacteria tuberculosis complex (MTBC). Most often involving the lungs, TB is transmitted by cough, with an infectious dose of less than 10 bacteria.1 Case fatality rates in untreated active pulmonary TB approach nearly 60%.2 Major medical advances of the past half-century have brought effective treatment capable of cure in nearly all identified cases.3,4 Despite this TB causes hundreds of thousands of deaths worldwide every year. The morbidity and mortality burden of TB is not uniformly distributed throughout the globe but rather disproportionately affects those living in poverty and those from resource-limited settings.5
EPIDEMIOLOGY AND MICROBIOLOGY
In this section, aspects of the epidemiology and microbiology of tuberculosis are presented.
 GLOBAL BURDEN OF TB AND RECENT PROGRESS
GLOBAL BURDEN OF TB AND RECENT PROGRESS
In 1990, the World Health Organization (WHO) declared TB a global emergency and in response, developed the directly observed therapy strategy (DOTS), promising to “Stop TB” by finding and treating infectious cases in resource-limited settings.6,7 Within a few years of its design, the World Bank labeled the DOTS strategy the most cost-effective health-intervention ever deployed and by 2012, an estimated 51 million people had been treated and an estimated 20 million lives saved.8–10 Since 2004, TB incidence is falling in all WHO regions and in each of the 22 highest burden countries.
Although global TB incidence is falling, TB remains a leading cause of global infectious mortality, second only to HIV infection.11,12 There were 8.7 million new cases of TB and 1.4 million deaths globally in 2011. Moreover, many TB deaths occur in young, previously healthy adults, and as such, TB is a top 10 cause of lost disease adjusted life years (DALYs).7
Broadly speaking, there are three major threats to global TB control: (1) Poor social conditions including inadequate housing and nutrition,5 (2) Immune compromise related to the HIV pandemic,2,13 and (3) Emergence of drug-resistant TB.14,15
 POVERTY AND TB
POVERTY AND TB
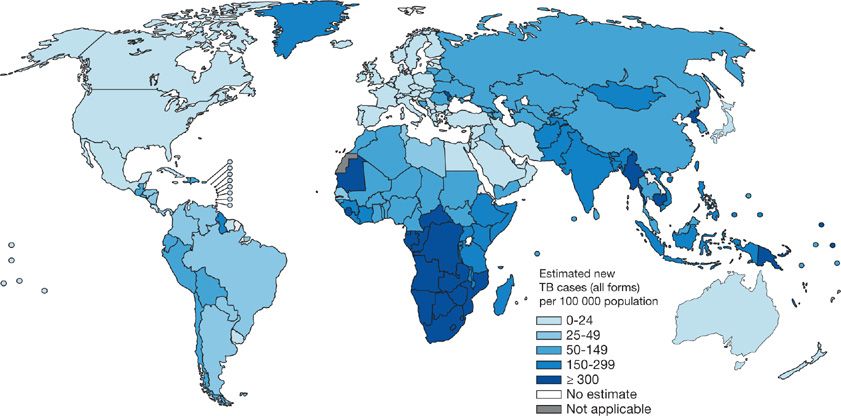
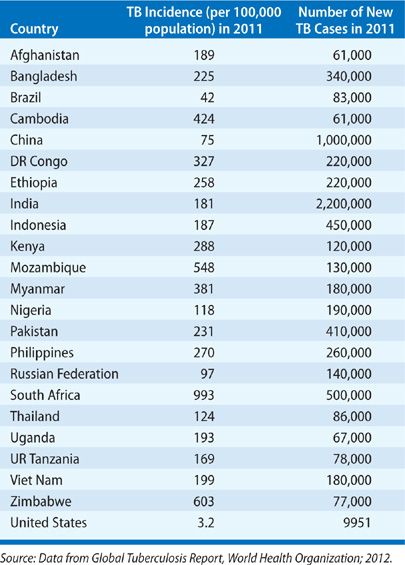
Globally, TB distribution correlates closely with poverty and human development indices.16–19 Population health factors such as water sanitation, childhood immunization rates, and life expectancy also independently predict TB incidence.20,21 A corollary of these relationships is that only 1% of the global TB burden occurs in the industrially developed countries of North America and Europe while more than 90% arises in Asia and Africa (Fig. 131-1) (Table 131-1).10
Figure 131-1 Estimated TB incidence by Country 2011. (Reproduced with permission from 2012. Global Tuberculosis Report, World Health Organization.)
TABLE 131-1 TB Incidence and Incidence Rate in 2011 for the 22-High TB Burden Countries and the United States
 HIV AND TB: CO-PANDEMICS
HIV AND TB: CO-PANDEMICS
HIV-induced immune suppression increases the risk of progression to active TB nearly 100-fold.22–26 Worldwide, TB is the most common opportunistic illness and a leading cause of death in patients with AIDS.27 HIV coinfection complicates 13% of all TB cases worldwide.10 As both a potent and common risk factor, HIV contributes substantially to TB incidence, especially in Sub-Saharan Africa.2,13
 DRUG-RESISTANT TB
DRUG-RESISTANT TB
In 2011, there were over 600,000 prevalent cases of multidrug-resistant (MDR) TB, defined by resistance to the two most important first-line antimicrobials, isoniazid and rifampin.10,28,29 MDR-TB accounted for approximately 3.7% of newly diagnosed TB cases and 20% of relapsed cases globally. Reliable global estimates of MDR-TB are difficult to obtain since rigorous drug-susceptibility testing (DST) is not available in most resource-limited settings endemic for TB.28,30 Eastern European countries have particularly high rates of MDR-TB; a phenomenon considered linked to the underinvestment in national TB programs and the social disruption that occurred following the collapse of the Soviet Union in the 1990s.31 MDR-TB is 100 times more costly to manage than is drug-susceptible TB, requiring prolonged courses of expensive and difficult to procure second-line drugs.32 In South Africa, MDR-TB prevalence is 3.2% but associated treatment costs account for one-third of the entire national TB program budget.32 Treatment outcomes are substantially worse in MDR-TB.29 Extensively drug resistant TB, or TB resistant to all currently available bactericidal agents, has been reported in nearly 60 countries.29,30 The increasing prevalence of MDR-TB is particularly chastening because it is entirely a “man-made” phenomena.31,33 With better management of TB programs, the emergence of resistance can be prevented and prevalence of MDR-TB can be reduced.14,15
 NEWER THREATS TO GLOBAL TB CONTROL
NEWER THREATS TO GLOBAL TB CONTROL
Diabetes, smoking, and malnutrition can also impair immunologic containment of TB infection. Their prevalence continues to increase globally and the cumulative impact may well threaten global TB control in the future.8,17,34
 TB EPIDEMIOLOGY IN THE UNITED STATES: HISTORICAL PERSPECTIVE
TB EPIDEMIOLOGY IN THE UNITED STATES: HISTORICAL PERSPECTIVE
In the first half of the 20th century TB was a leading cause of morbidity and mortality in the United States. In 1920 pulmonary TB accounted for nearly 1 in 13 of all deaths, outranking cancer.6,35,36 Improvements in social conditions and population health during the early 20th century led to significant reductions in TB incidence and, with the introduction of effective chemotherapy in the 1950s, incidence declined even further.4 By the 1970s, TB incidence in the United States had reached historical lows, less than 10 cases per 100,000 people and TB seemed on the brink of elimination. Unfortunately, public health funding for TB control was then greatly reduced and by the mid-1980s a nationwide resurgence ensued, particularly in the major urban centers.6,37–39 It is estimated that during the 1980s and early 1990s, when TB funding in the United States was nearly eliminated, 64,000 excess cases occurred.37,40
 CURRENT US TRENDS IN TB EPIDEMIOLOGY
CURRENT US TRENDS IN TB EPIDEMIOLOGY
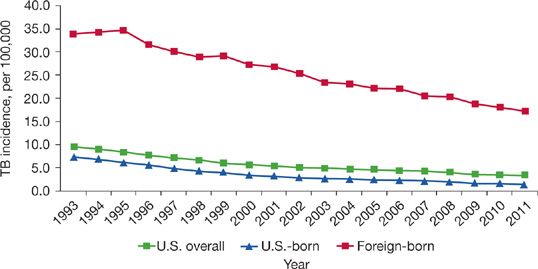
With renewal of Government investment in the mid-1990s TB control was reestablished and TB rates have since declined every consecutive year in the United States (Fig. 131-2).41 By 2012, there were less than 10,000 total reported cases of TB in the United States, or just 3.2 cases per 100,000 people, the lowest rate ever reported.41
Figure 131-2 TB Case Rates in US-born versus Foreign-born persons, United States, 1993 to 2011. (Reproduced with permission from Frieden T. Reported Tuberculosis in the United States, 2011. CDC; 2012).
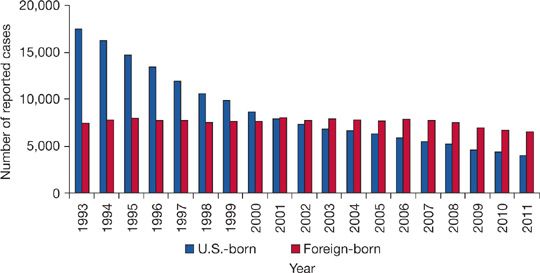
TB remains a concern within specific demographic groups. The foreign born, especially newly arrived immigrants, account for an increasing proportion of TB cases in the United States, representing nearly two-thirds of all US cases in 2012 (Fig. 131-3).41 Mexico (22%) and the Philippines (12%) are the most common countries of origin of imported TB.42,43 In foreign-born US residents, TB usually represents reactivation of infection remotely acquired in TB-endemic countries prior to arrival rather than new infection resulting from local transmission.43
Figure 131-3 Number of TB cases in US-born versus Foreign-born persons United States, 1993 to 2011. (Reproduced with permission from Frieden T. Reported Tuberculosis in the United States, 2011. CDC; 2012).
Among the US born, TB burden is concentrated in indigenous peoples, incarcerated populations, and the unstably housed.40,43–46 In these groups, “mini” outbreaks of TB resulting from recent transmission occur with some regularity, especially in major urban centers. Several authors have shown that race, substance abuse, malnutrition, and socioeconomic status are each independently correlated with TB incidence in the United States.18,47–51
Childhood TB incidence reflects ongoing TB transmission and as such, is important indicator of TB control within a community. In the United States, 80% of childhood TB occurs in US-born children, and mostly those from racial and ethnic minority groups.43
 MICROBIOLOGY OF TUBERCULOSIS
MICROBIOLOGY OF TUBERCULOSIS
Mycobacterium tuberculosis as the causal agent of TB was first conclusively demonstrated in 1882 by Robert Koch and acknowledged with a Nobel Prize in 1905.52
M. tuberculosis is a slender, slightly curved, rod-shaped bacterium (or bacillus) averaging 3 by 0.3 μ in size and visible by light microscopy. It is nonmotile, nonencapsulated, and nonspore forming. M. tuberculosis is strictly aerobic.53,54 Traditionally, mycobacteria are grown on solid, enriched media where rough, pigmented colonies generally appear 4 to 6 weeks after inoculation. MTBC can also be cultivated in specialized liquid media where characteristic “cords” visible by light microscopy are formed. Rapid liquid culture systems (e.g., BACTEC) have been adapted for use with mycobacteria and shorten time to detection of growth to a little as 9 to 16 days, depending on the initial concentration of the bacteria in the specimen tested.55,56
MTBC: Eight closely related mycobacterial species capable of causing human TB have been identified and together they comprise the MTBC: M. tuberculosis (Koch bacillus), M. bovis (the agent of bovine TB), M. caprae (bovine TB), M. africanum (a not infrequent cause of human TB in West Africa), M. microti (agent of rodent TB), M. pinnipedii (agent of TB in seals and a rare zoonotic disease in marine biologists attending to them), and M. canettii (an ancient bacillus and rare cause of human TB in East Africa) (Table 131-2).55,57,58
TABLE 131-2 Members of the Mycobacterium Tuberculosis Complex (MTBC)a
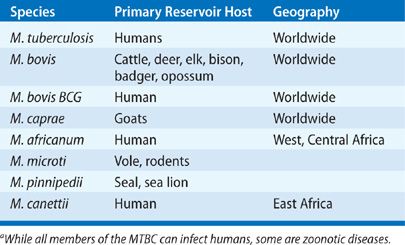
M. tuberculosis causes the vast majority of TB today. In the early 20th century, M. bovis accounted for up to 20% of all cases of human TB but with modern surveillance of animal herds and widespread use of milk pasteurization, M. bovis now causes less than 1% of reported TB cases in the United States.59,60 Sporadic outbreaks of M. bovis infection still occur in the United States, and ongoing surveillance is important for food-chain safety. Human-to-human transmission of M. bovis has been demonstrated.61
 MYCOLIC ACID AND THE MYCOBACTERIUM GENUS
MYCOLIC ACID AND THE MYCOBACTERIUM GENUS
The MTBC are members of the diverse Mycobacterium genus. The outstanding feature of the mycobacteria is their lipid-rich cell wall containing very high concentrations of mycolic acids.53,54,56,62 This cell wall prevents reliable uptake of Gram stain. With phenol additive mycobacteria can eventually be stained to facilitate light microscopy. Once stained, removal of dye is extremely difficult, resisting even acid alcohol wash (hence “acid-fast bacilli” [AFB]). Lipids in the mycobacterial cell wall also help resistance to drying, a property that allows MTBC to retain infectiousness even within dried droplet nuclei. The metabolic investment required to produce mycolic acids significantly slows growth rate56and prolongs the time to detection in incubated clinical specimens submitted to laboratory.53 The mycolic acid containing cell wall of mycobacteria is also a major virulence factor, helping mycobacteria avoid innate immune defenses and intracellular killing by nonactivated host phagocytes.63
 COMPARISON TO OTHER MYCOBACTERIA
COMPARISON TO OTHER MYCOBACTERIA
The nontuberculous mycobacteria (NTM) are free-living saprophytic bacteria, commensal inhabitants of the soil and/or aquatic environments (see Chapter 132). The NTM are nontransmissible under ordinary conditions, and only rarely infect humans as opportunists. In contrast, MTBC are transmissible, obligate primary pathogens without an environmental reservoir.55
 TRANSMISSION
TRANSMISSION
Important considerations in the transmission of tuberculosis are discussed below.
Droplet Nuclei
In the late 1930s, William Wells, sanitation engineer at Harvard University first conceived of “droplet nuclei” for airborne transmission of infectious diseases.64 He found that forceful expiratory efforts such as coughing and sneezing, discharge minute respiratory droplets of sputum containing viable bacilli that, when increasingly reduced in size by evaporation, become infectious droplet nuclei, each measuring less than 5 μ.65 Certain healthcare procedures, for example, bronchoscopy, sputum induction, autopsy, and even irrigation of abscesses, also produce infectious droplet nuclei.66
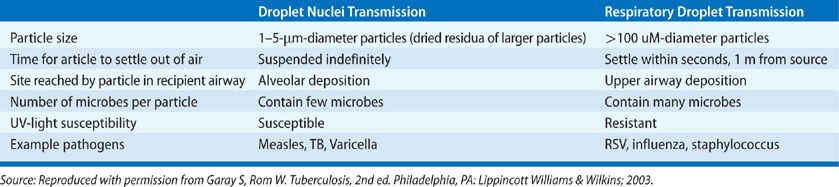
Due to small size, droplet nuclei have an extremely slow settling rate in air (0.5 mm/s or less). This permits their transport by air currents for significant distances. Larger respiratory droplets, on the other hand, settle out of the air quickly, and travel only a few feet from the source case. The microbes suspended within droplet nuclei are highly susceptible to germicidal levels of ultraviolet light.2,67 The small size of droplet nuclei also facilitates penetration of the bronchial defenses allowing access to terminal alveolar macrophages. The fairly tortuous and branched path of the pharynx and bronchial tree ensures that larger respiratory droplets are instead deposited on the mucosal lining of the airway or trapped in the mucociliary elevator and expelled (Table 131-3).65
TABLE 131-3 Key Distinguishing Features of Droplet Nuclei And Respiratory Droplets
Airborne Transmission of TB
In the 1950s, Richard Riley, a medical student working with Wells, elegantly demonstrated that TB is near exclusively transmitted by droplet nuclei. Riley commandeered a TB ward of six rooms for his study. The air from each individual room was ventilated to an upper chamber, where caged guinea pigs were kept, sometimes passing through an adjustable UV light source.68 Guinea pigs are considered uniquely susceptible to infection with M. tuberculosis, even by dilute aerosols, and their use allowed quantification of degree of patient infectiousness.
Relatively few respiratory diseases are preferentially transmitted via the airborne route; these include TB, measles, varicella, and smallpox. Most infectious respiratory diseases (including, e.g., pertussis, influenza, the common cold, and pneumococcal pneumonia) are instead transmitted by direct contact with larger respiratory droplets leading to colonization of the host nasopharynx before subsequent lung invasion.
Risk Factors for TB Transmission
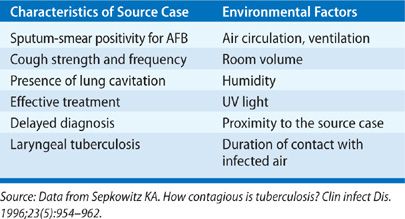
The risk of TB infection is a function of exposure to the tubercle bacilli. In turn, this depends on the interaction of three factors: infectiousness of the source case, environmental conditions that impact droplet nuclei concentration per volume of air, and duration of contact with the source (Table 131-4).
TABLE 131-4 Factors Associated with Number of Infectious Droplet Nuclei per Volume of Air and TB Transmission
Source Case Infectiousness
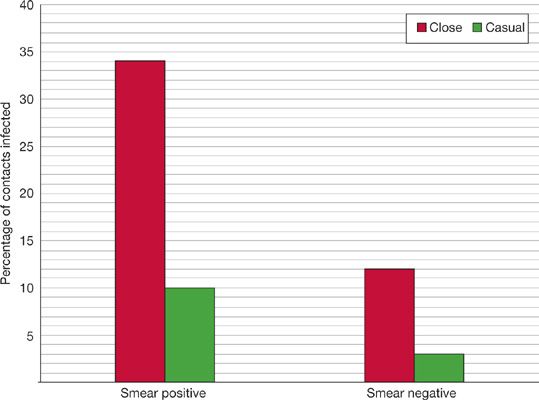
An important finding from Riley’s studies was the extreme variability in infectiousness of TB patients.69,70 The dominant predictors of infectiousness in source cases are the presence of cough, lung cavitation on chest radiograph, and acid-fast bacilli visible in sputum by smear microscopy. Sputum-smear microscopy positive cases excrete about 108 bacilli per mL of sputum compared to less than 103 bacilli per mL of sputum in smear-negative cases.66 About 35% of close contacts of sputum smear–positive patients will become infected, compared to less than 10% from sputum smear–negative cases (Fig. 131-4).66,71–73
Figure 131-4 Infectiousness of tuberculosis by bacteriologic status of and proximity to source case. (Reproduced with permission from Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc. 1975;50(1):90–106).
Riley also demonstrated that effective anti-TB treatment could rapidly render patients noninfectious, usually within a few days of initiation.74 The implications for discontinuing hospital isolation are somewhat controversial.75 As a matter of convention, pulmonary TB patients are usually considered noninfectious after 2 weeks of effective chemotherapy, provided drug resistance and nonadherence are excluded.76
Conducive Environment
Important environmental factors influencing TB transmission include ventilation (or room air changes per hour) and ultraviolet light.66,77,78 Proximity and duration of contact with source case are also determinants of TB transmission. Household contacts are several times more likely to be infected than are casual contacts from the community.66,72 Thus crowded housing with poor ventilation and inadequate natural lighting – such as occurs in prisons, homeless shelters, American inner-city housing projects, and the large urban slums of the developing world – are particularly conducive for TB spread.16,79
Host Susceptibility
Genetic determinants of innate immunity are likely important host susceptibility factors but are poorly understood.63,80 Some studies suggest that previous TB infection (as manifested by tuberculin skin test positivity) may partially protect against reinfection, although the degree of protection has not been quantified.81–83 Vaccination with attenuated M. bovis Bacille Calmette-Guérin (BCG) strain probably does not reduce the risk of infection with MTBC, even if it does reduce the subsequent chance of developing disseminated active disease once infected.66,80,84
NATURAL HISTORY AND PATHOPHYSIOLOGY OF TB INFECTION
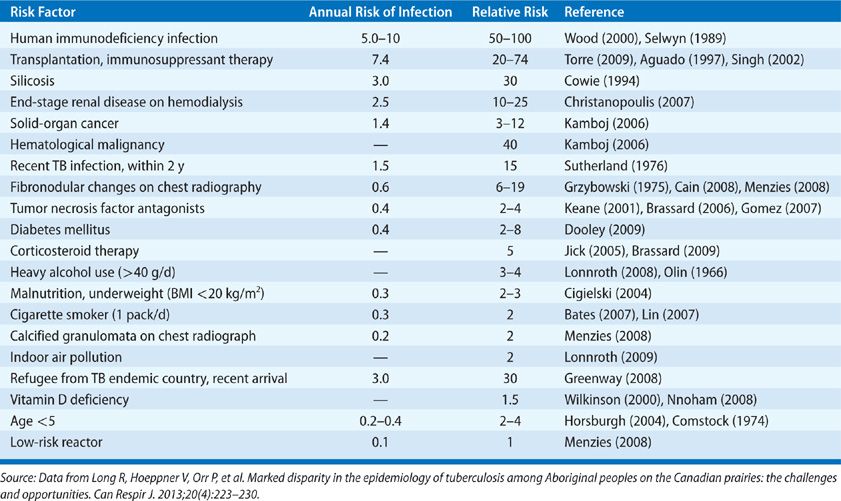
It is estimated that one-third of all humans are infected with TB.85 However, only a small fraction of the individuals within this massive reservoir ever develops active TB; most infections remain latent without apparent ill effect on the host. Risk factors associated with progression to active disease once infected are shown in Table 131-5.
TABLE 131-5 Risk Factors for Progression to Active TB in Those Latently Infected. Low-Risk Reactor—TST Positive with no Known Risk Factor, Normal Chest Radiograph
 FROM EXPOSURE TO INFECTION AND THE INNATE IMMUNE RESPONSE
FROM EXPOSURE TO INFECTION AND THE INNATE IMMUNE RESPONSE
The natural history of TB infection is outlined in Figure 131-5. Following exposure to an infectious source case, many contacts do not become infected, and will not convert their TST—either because infectious droplet nuclei did not reach their terminal alveoli or because any successful invaders were immediately cleared by intrinsic microbicidal activity of macrophages.3
Figure 131-5 Natural history of TB infection. pTB, pulmonary tuberculosis; TST, tuberculin skin test. (Reproduced with permission from Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125(3 Pt 2):8–15).
However, in some individuals, invading mycobacteria engulfed by alveolar macrophages manage to evade intracellular killing. Mycobacteria bypass innate immune mechanisms and replicate without limitation, spreading to regional lymph nodes, and silently disseminate hematogenously.1,57,80 After a few weeks, dendritic cells are activated and produce cytokines such as tumor necrosis factor alpha (TNF-α) that recruit blood-borne monocytes and lymphocytes to sites of infection, setting the stage for the adaptive cellular immune response.80
 THE ADAPTIVE IMMUNE RESPONSE AND CONTAINMENT OF MYCOBACTERIAL INFECTION
THE ADAPTIVE IMMUNE RESPONSE AND CONTAINMENT OF MYCOBACTERIAL INFECTION
The main effectors of the adaptive, cell-mediated immune (CMI) response to mycobacteria are the CD4-positive subset of T lymphocytes.80 Once stimulated, T lymphocytes release their own battery of cytokines (including interferon-gamma [IFN-γ]) that serve to reciprocally activate infected macrophages and trigger-enhanced intracellular mycobacteria killing. This is the basis for the tuberculin skin test and IFN-γ release assays, which become positive with the development of a host CMI response. Over the next few weeks, the CMI directs necrotizing granuloma formation, which usually contains bacillary replication.
 PRIMARY DISEASE
PRIMARY DISEASE
About 5% of immune competent individuals do not control initial mycobacterial replication and instead progress to primary TB disease, usually within 18 months. The risk of progression to primary disease is even higher in those with compromised CMI or other risk factors. Primary disease may occur at the initial site of lung entry (typically the mid and lower lung zones where greater airflow directs droplet nuclei deposition), regional lymph nodes, or rarely at metastatic sites initially seeded during early occult hematogenous dissemination.
 REACTIVATION DISEASE FROM LATENT INFECTION
REACTIVATION DISEASE FROM LATENT INFECTION
For the individuals who successfully contain the initial infection and avoid primary disease, mycobacteria lie latent within healed, fibrotic and/or calcific granulomata. At this stage, mycobacteria cannot be cultured from host sputum or tissue specimens and symptoms are not present. It is believed that latent tuberculous infection (LTBI), with the potential for future reactivation, persists for life of the host.86
In a small minority, granulomas break down, mycobacteria replication increases, and symptomatic disease develops. Such reactivation can occur with age-related immune senescence or in those with acquired risk factors for active TB. Reactivation most commonly occurs in the apical-posterior segments of the lungs, where higher oxygen tension favors bacillary replication, but disease can occur at any previously seeded site.1 Once reactivated, bacilli can usually be cultured from sputum and/or tissue samples.
 CASEATION AND CAVITATION
CASEATION AND CAVITATION
Differences in quality of host adaptive immune response determine clinical presentation.57,80 Some individuals develop a particularly robust, caseating granulomatous inflammatory response with resultant tissue destruction and lung cavitation—they discharge large amounts of bacilli into airways and are highly contagious. At the other end of the spectrum, a severely weak or immature granulomatous response allows hematogenous dissemination of bacilli, accompanied by widespread inflammatory foci of poorly formed granulomas. Each focus typically enlarges to about 3 mm in size, or about the size of millet seed. This severe form of TB (miliary TB) has a high case-fatality rate.
CLINICAL MANIFESTATIONS
TB had had a number of illuminating vernacular names through history. In ancient Greece, TB was called “phthisis” meaning to waste away. And in Rome, “tabes” was used, indicating wasting and overall decay. “Consumption,” a term applied to TB in 19th century England is particular evocative, referring to the observation that TB sufferers appear to be gradually “consumed” by the disease, becoming lighter and less robust over months.87
 SITE OF DISEASE AND CLINICAL PRESENTATION
SITE OF DISEASE AND CLINICAL PRESENTATION
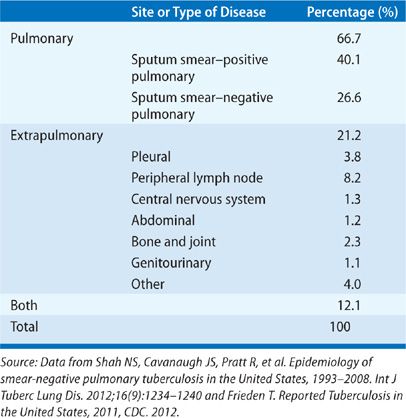
TB is predominantly a respiratory disease, affecting the lungs in about 80% of cases (Table 131-6).43,88 About 30% of TB cases involve an extrapulmonary site, occurring either with or without concomitant lung involvement. TB can affect virtually any organ, although peripheral lymph nodes and pleural space are the most common extrapulmonary sites.89
TABLE 131-6 Proportion of Reported TB Cases in the United States, by Predominant Site of Disease
Classically TB presents insidiously over weeks, with persistent local symptoms correlating to granulomatous inflammation (e.g., cough in the case of pulmonary TB, neck mass for cervical lymph node TB) plus constitutional symptoms that correlate to the production of pyrogenic cytokines such as TNF-α (e.g., fevers, night sweats, anorexia, weight loss). Initially the cough may be dry but after several months becomes productive. Fever may be absent, especially in the elderly.90 With advancing disease, hemoptysis, anorexia, and weight loss can occur.91–93 Importantly, some patients are asymptomatic even with clear TB disease activity demonstrable on culture or radiography—a prospect that frustrates TB control program efforts at active case finding.3,94
 PHYSICAL EXAMINATION
PHYSICAL EXAMINATION
Even when relatively extensive disease is present, pulmonary TB most often produces no detectable abnormality on physical examination.91,94 It is important to examine for signs of extrapulmonary disease such as lymphadenopathy, abdominal, or bone and joint involvement, particularly in HIV-infected individuals. Tachypnea and hypoxia are relatively rare except with extensive lung destruction or miliary disease.
 CHEST RADIOGRAPHY IN PULMONARY TB
CHEST RADIOGRAPHY IN PULMONARY TB
Radiographic findings of TB are well described.94–96 Sensitivity for active TB is 70% to 80%, and even less in patients with HIV or other severe immune compromise. Further, chest radiography is only moderately specific and there is generally poor agreement between chest film readers.97–99 Chest radiography alone also cannot reliable distinguish between active and healed, latent TB.97,98,100
Typical chest Radiograph Patterns
Four radiographic features suggest active TB: (1) Nodular opacities located in the apical-posterior segments of the upper lobes or superior segment of the lower lobes, (2) associated volume loss and fibrosis, (3) lung cavitation, and (4) endobronchial spread. Endobronchial spread to dependent lung segments fills lung acinar units, resulting in 4- to 5-mm size nodular opacities on plain film (acinar shadows) and a “tree-and-bud” pattern on computed tomography (CT) (Figs. 131-6 and 131-7).95,98,101
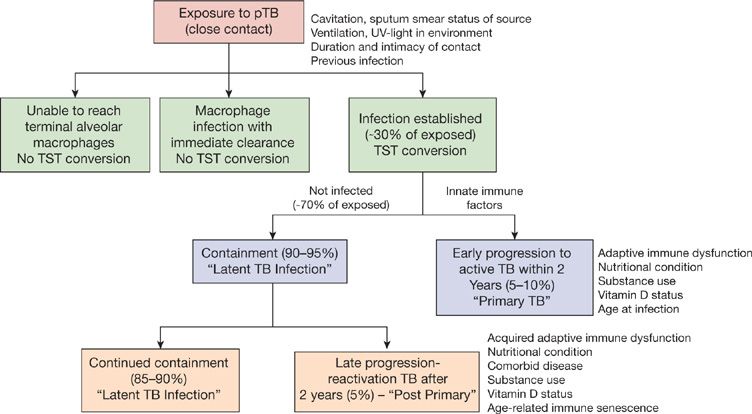
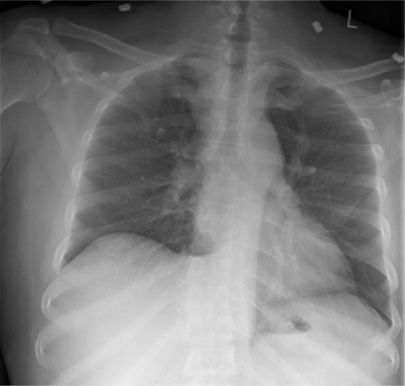
Figure 131-6 This 32-year-old male refugee recently arrived from Eritrea. He presented with persistent cough and fevers despite completing two courses of antimicrobial therapy directed against bacterial community–acquired pneumonia. Sputum sample was both smear positive and culture positive for MTBC.
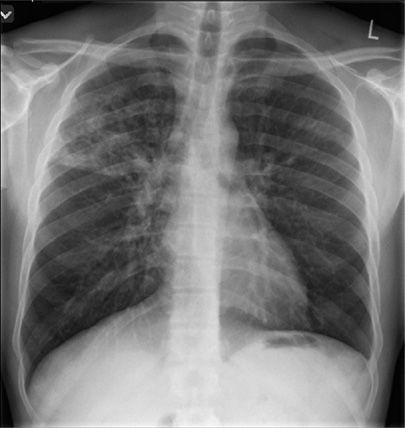
Figure 131-7 This 36-year-old man Aboriginal man with history of homelessness. This radiograph demonstrates typical manifestations of reactivation TB: upper lobe nodular opacities, cavitation, acinar shadowing from endobronchial spread, and fibrosis.
Atypical Chest Radiograph Patterns
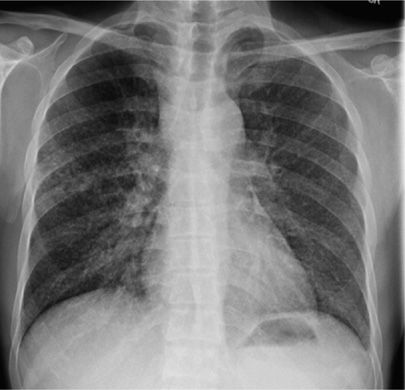
Atypical radiographic patterns are seen in children, the elderly, the immunocompromised, and those with primary disease.96,100,102 Atypical features include lower lung zone infiltrates without cavitation, unilateral pleural effusion (Fig. 131-8), and ipsilateral hilar or mediastinal adenopathy (Fig. 131-9). Intrathoracic TB lymphadenitis is often better appreciated on CT where lymph node enlargement, rim enhancement, and low central attenuation are characteristic.95
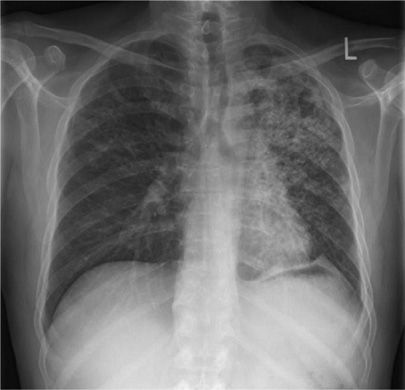
Figure 131-8 This 22-year-old man identified as a close household contact of a smear-positive case of pulmonary tuberculosis. Seven weeks later he developed fevers and cough. This radiograph demonstrates unilateral pleural effusion with compressive atelectasis. Diagnostic thoracocentesis revealed a transudate with cellular infiltrate, predominantly lymphocytic. Sputum was culture negative for mycobacteria but pleural specimen eventually grew MTBC.
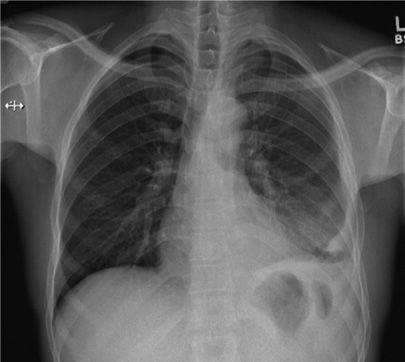
Figure 131-9 This 42-year-old HIV+ man with a CD4 count of 150 cell/mm3 presented with cough. Significant mediastinal and right hilar adenopathy are present without apparent lung parenchymal disease. Sputum cultures were smear negative but culture positive for MTBC.
Miliary TB is rare but when present, produces a distinctive, readily recognizable radiographic pattern: Innumerable, interstitial nodules uniformly distributed throughout all lung fields, without reduction in lung volumes (Fig. 131-10).
Figure 131-10 This 36-year-old HIV+ man presented with fevers. Chest radiograph identified right upper lobe infiltrate and a background miliary pattern consisting of innumerable noncalcified nodules measuring 2 to 3 mm, randomly distributed.
DIAGNOSIS OF TB
Most US clinicians will practice for years without ever encountering a case of TB. For every 450 ambulatory visits for community-acquired pneumonia (CAP), and for every few dozen cases of lung cancer, there is on average one case of TB in the United States.103,104 In low-incidence settings, many patients diagnosed with TB will have experienced multiple contacts with the healthcare system before the diagnosis of TB is considered.105,106
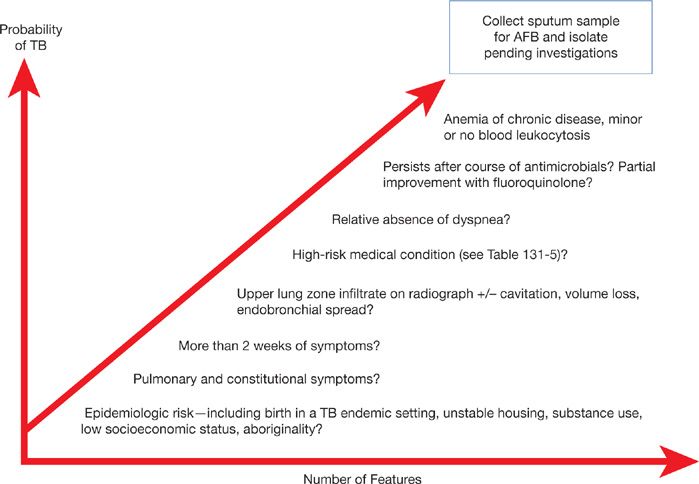
Thus the diagnosis of TB requires a very high-index of suspicion considering epidemiologic risk factors and suggestive clinical–radiographic features (Fig. 131-11). Ultimately, the definitive diagnosis requires culture confirmation.
Figure 131-11 Clinical factors increasing index of suspicion for tuberculosis. Increasing probability of TB warrants further investigation, especially collection of respiratory samples for mycobacterial stain and culture. For hospitalized patients, or those in congregate living settings, increasing suspicion warrants prompt isolation pending results of investigations. (Used with permission of Richard Long, unpublished observations).
 LABORATORY INVESTIGATIONS
LABORATORY INVESTIGATIONS
Anemia is commonly observed in TB, usually due to chronic inflammatory state or malnutrition.94 Syndrome of inappropriate ADH can complicate pulmonary or central nervous system TB.107 Historically, disseminated TB was an important cause of adrenal insufficiency. Hypercalcemia is a relatively frequent complication of TB.108 Activated macrophages within granulomas upregulate 1-alpha-hydroxylase, which can in turn activate vitamin D and lead to increase in calcium absorption.109–111 Hypercalcemia of TB can be symptomatic and occasionally leads to nephrocalcinosis, nephrolithiasis, or acute volume depletion. Vitamin D supplementation during TB therapy may increase the risk of hypercalcemia.112
Specimen Collection
Protocols to ensure proper specimen handling, storage, transport, and labeling are available.94 Ideally, specimen collection should occur before initiation of therapy to increase yield.
For the diagnosis of pulmonary TB at least three sputum samples, each 5 to 10 mL, should be collected at least 1-hour apart. For those patients unable to expectorate spontaneously, sputum induction (with nebulized hypertonic saline) or bronchoalveolar lavage (BAL) is an alternate established method.113 Induced sputum samples produce slightly higher yield than do BAL samples (87% vs. 73%), and sputum induction is much better tolerated, less invasive, and lower cost.114,115 If bronchoscopy is performed to evaluate other diagnostic considerations, then an additional sputum sample collected immediately after bronchoscopy may have particularly high-yield for mycobacterial identification.113
For diagnostic confirmation of extrapulmonary TB, tissue or fluid samples should be submitted fresh (or in sterile normal saline) without addition of preservative (e.g., formalin) because this will prevent subsequent identification, culture, and DST in the microbiology laboratory. It is important to notify both the laboratory and clinician before collecting a specimen if extrapulmonary TB is suspected to allow the laboratory to take appropriate biosafety precautions during specimen handling to prevent laboratory transmission of TB.
Direct Microscopy for AFB
Sputum-smear microscopy is more than 110 years old but remains the most widely used investigation for active TB today.52 Two methods are commonly used for acid-fast staining of mycobacteria: carbolfuchsin (e.g., Ziehl–Neelsen [ZN]) and fluorochrome-based procedures (e.g., auramine–rhodamine dye). Fluorescent methods slightly improve sensitivity over ZN and also allow the use of lower microscopic magnification during inspection of the slide, significantly shortening the time required to examine an entire specimen.116
Sputum microscopy is widely available and low cost. It is performed directly on clinical specimens allowing rapid turn-around-time, thus facilitating prompt recognition, isolation, and treatment of infectious TB cases. Microscopy also provides prognostic information: Smear-positive TB cases are more infectious and have much higher case-fatality rates if untreated than do sputum smear–negative cases.72,117,118
However, sputum-smear microscopy has well-known limitations. Sensitivity for pulmonary TB is only about 60% to 70%, and even lower in extrapulmonary specimens and in those with HIV coinfection. That NTM also stain acid-fast lowers the specificity of smear microscopy.119–121 In low TB prevalence settings, NTM are recovered from about 30% to 50% of AFB smear–positive sputum samples.
Mycobacterial Culture
Mycobacterial culture remains the gold standard for the diagnosis of active TB. About 5000 to 10,000 bacilli per milliliter of specimen are required for detection by smear microscopy and about 100 bacilli per millimeter for nucleic acid amplification (NAAT); culture methods can detect as few 10 viable bacilli per millimeter of sample.94 In addition, biochemical and phenotypic testing of isolated organisms provides near perfect specificity, distinguishing even between individual members of the MTBC. Of particular importance, isolation of the infecting organism permits DST and is required to conduct molecular subtyping (i.e., DNA fingerprinting).
Culture of MTBC from clinical specimens takes, on average, between 2 to 4 weeks, but as long as 8 weeks in some specimens with low initial concentration of organism.122 Culture requires a Level III biosafety laboratory to prevent transmission to laboratory technicians and availability of such facilities is poor in most high-burden, resource-limited settings.
Nucleic Acid Amplification
NAAT is a useful tool in the diagnosis of TB offering higher sensitivity than microscopy and shorter turn-around-times than culture.100,123,124 Sensitivity of NAAT in AFB smear–positive respiratory samples is excellent, usually greater than 95%.123 However, in paucibacillary specimen types, such as AFB smear–negative sputum and extrapulmonary samples, the sensitivity is reduced to 40% to 60%, with a negative predictive value inadequate to exclude a diagnosis of TB.125–129 Specificity of NAAT methods is very high, ranging between 90% and 100% for all specimen types.
Performance of traditional NAAT requires sophisticated laboratory facilities. Recently, an automated in-cartridge assay (Xpert/MTB-RIF [Cepheid, Sunnyvale, California]) was developed to allow NAAT directly from unprocessed clinical specimens, without the need for centralized reference laboratory, even in resource-limited settings.130–132 The Xpert/MTB-RIF simultaneously detects mutations within the mycobacterial RNA-polymerase gene that correlate with rifampin-drug resistance, thus identifying MDR cases several weeks before culture-based testing. The WHO has signaled its intent to deploy this tool in high-burden, resource-limited settings throughout the world.132
Testing for Latent TB infection
There are two main tests for the identification of LTBI: (1) Tuberculin skin test (TST) and (2) IFN-γ release assays (IGRAs). Both are indirect, in that they evaluate the presence of host cell–mediated immunity rather than detect actual mycobacterial organisms or antigens.133 As such, neither can distinguish active disease from latent infection. Both tests have very poor sensitivity and predictive value for active TB and use in this context is discouraged.134,135 These tests are better used to assess candidates for LTBI treatment (discussed below).
MANAGEMENT OF ACTIVE TB
Prior to the availability of chemotherapy, prevailing wisdom prescribed fresh air, nutrition, sunlight, and rest for treatment of TB. In 1947, clinical outcomes of smear-positive pulmonary TB patients treated at a sanatorium in the United Kingdom were recorded: within 4 years of entry, 55% died, 20% remained chronically affected, and 25% experienced remission.136 With the discovery of chemotherapy for TB, mortality dropped orders of magnitude and within a decade TB sanatoria were shuttered.6,137
 PRINCIPLES OF TB THERAPY
PRINCIPLES OF TB THERAPY
The aims of TB therapy are to (1) Interrupt transmission by rapidly rendering patients noninfectious, (2) relieve symptoms and prevent mortality, (3) prevent the emergence of drug resistance, and (4) prevent future relapse by providing a definitive cure.
To achieve these aims, treatment regimens must include combination of potent bactericidal drugs which are provided for a minimum of 6 months. The choice of regimen should be guided by the results of DST. TB regimens are divided into an initial intensive phase, designed to quickly reduce large bacillary burden, followed by a prolonged continuation phase that consolidates antimycobacterial killing while allowing intermittent dosing and lower pill-burden. A high level of adherence is required to prevent relapse and emergence of resistance. Rifamycins have a critical role in preventing relapse and whenever possible should be provided throughout the treatment. Several randomized clinical trials have established standardized treatment regimens as outlined in Table 131-7. Reported success rates with these regimens approach 95%.138–140
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree