Fig. 7.1
A metallic (a) and single-use (b) pleural needle. The pleural trocar, 2–3 mm in diameter, 100 mm in length, with tap. A pointed obturator to penetrate the skin and the intercostal tissue and subsequently changed to a blunt obturator to pass through the parietal pleura
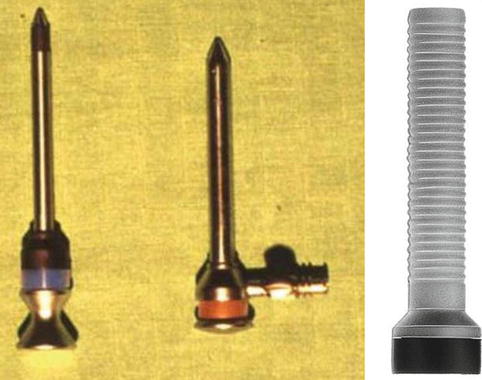
Fig. 7.2
Trocars. From the left to the right. 7-mm diameter metallic trocar consisting of an obturator and a cannula. 5-mm diameter insulated trocar allowing the use of coagulating forceps. 10-mm diameter plastic trocar

Fig. 7.3
A direct-viewing (0°) and angle-viewing (50° oblique angle of view) optical telescope. (a) Examination telescope, Panoview, 7 mm in diameter and 350 mm in length, allows for high-quality exploration. (b) A new telescope is available which includes a working channel for suction catheter, grasping forceps, and coagulating forceps with a single point of entry (Richard Wolf, Knittlingen, Germany)
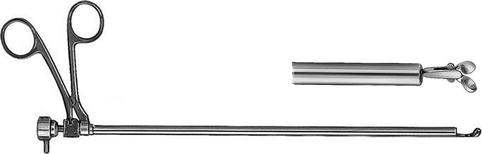
Fig. 7.4
Optical biopsy forceps. This is mainly used for biopsies of the parietal pleura. Coagulation is not possible with this instrument (Richard Wolf, Knittlingen, Germany)
Additional essential instruments include an insulated trocar (Fig. 7.2) that can be used to create a second port of entry and through which electrocautery can be performed. Recently new sets are available for single puncture thoracoscopy allowing minimally invasive diagnostic treatment with one small incision. With a 5.5-mm trocar, the procedure is possible under local anesthetics and without painful stress for the patient (Fig. 7.5).


Fig. 7.5
A new generation optical biopsy forceps with working channel for instruments including the coagulating forceps (Richard Wolf, Knittlingen, Germany)
The optimal diameter of the thoracoscope (trocar and telescope) is 7 mm (Fig. 7.3a), as originally developed by Boutin in conjunction with the Wolf Corporation (Wolf Company, Knittlingen, Germany) (Boutin et al. 1981b). Larger telescopes (diameter 10–12 mm) are available, but they have been developed with the surgeon in mind, where the procedure will be performed under general anesthesia and double-lumen intubation. However, their large size makes them impractical for a procedure performed under local anesthesia.
In addition, equipment with a smaller diameter is also available, the so-called minithoracoscopy (please refer to the dedicated chapter in Part VI of this textbook).
Both 3- and 5-mm optical biopsy forceps (Fig. 7.4) are available and frequently provide adequate biopsies for a definitive diagnosis of the underlying pathology.
7.2.2 Other Required Equipment
A suitable area for surgical scrubbing, sterile gowns, sheets, and gloves
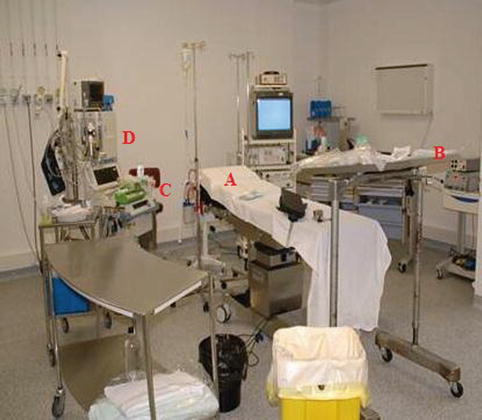
A thoracoscopy table
Separate mobile carts
A mayo stand, which can be covered with sterile sheets, for the instruments (Fig. 7.7)
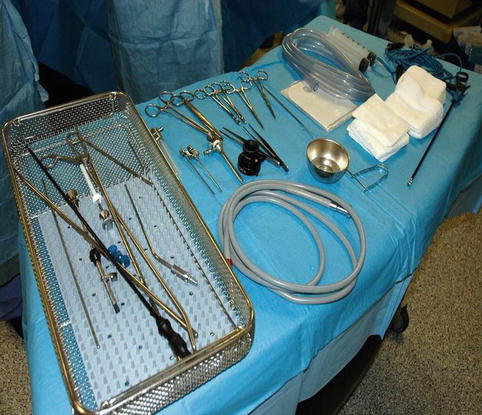
Fig. 7.7
A Mayo stand covered with sterile sheets for the instruments
Anesthetic equipment
Monitoring equipment including ECG, blood pressure, and saturation probes
Needles – 24G and 21G for the administration of local anesthetic agents
10- and 50-mm syringes
Surgical swabs
Scalpel
Clamps
Grasping forceps and a swab holder
Electrocautery and loop to divide adhesions and control hemorrhage – if it occurs
Sterile covers for the cables used to attach the optics and camera to the lighting source
Plastic sterile aspiration tubes, 4 and 6 mm in diameter
Aspiration tubing and collection bottles of at least 2 l capacity which can be connected to negative pressure
Cupulas for local anesthetic (LA), warm saline – used to prevent fogging of the optics – and soap
Chest drains ranging from size 20 to 32 Fr
A guide for the chest drain or a self-contained chest tube set with an inner stylet
7.3 Practical Considerations
It is mandatory that the operator is skilled with a good knowledge of pleural anatomy and the associated landmarks. The physician should be well acquainted with all the equipment used during the procedure. When setting up for the procedure prior to the commencement of the intervention, it is necessary to ensure that all necessary equipment is available. We advise that the drain and underwater seal is prepared and the patient is earthed for electrocautery before starting the intervention. This will allow for a rapid response to complications should they arise. In addition, the physician must be skilled in the postoperative management of patients including pain control and chest drain assessment and removal.
The light source should be of a high quality to maximize the quality of the images. Care must be taken to ensure that the connecting cables between the light and power sources and the thoracoscope are attached correctly. Sterility of these cables must be maintained at all times to reduce postoperative infectious complications. Although not essential for the procedure, we recommend that the physician invests in equipment that will allow the capture of images and/or movie clips. This provides an objective record of the pleural space especially if redo thoracoscopy is planned to access the response to therapy (Breen et al. 2008).
A compromise must be made when choosing the optimal trocar size. Large trocars allow for the insertion of large telescopes which will improve the quality of the procedure. However, the larger the trocar, the greater the discomfort to the patient. We believe that a 5- or 7-mm trocar is optimal for thoracoscopy (Fig. 7.2), allowing good visualization of the pleural space and biopsy through a single port of entry when using an optical forceps (Fig. 7.4). A conically shaped tip on the trocar reduces the risk of trauma to the intercostal neurovascular bundle during introduction.
The vast majority of procedures including talc poudrage can be performed through a single port of entry – if the initial trocar size is at least 5 mm in diameter. A second port of entry is occasionally indicated. This point of entry is located one intercostal space superior or inferior to the primary port of entry or, in the setting of hemorrhage, located to allow electrocoagulation of the site of bleeding
7.3.1 Second Port of Entry
A second port of entry is necessary in some scenarios. These include situations where the movement of the trocar is sufficiently painful to prevent a full inspection of the area of interest. This complication can be minimized by choosing the position carefully at the outset with the aid of available radiographs, CT, and pre-thoracoscopy ultrasound (see Chap. 4).
However, in some cases it may be due to simple geographical issues between the entry point and the lesion or secondary to a narrow intercostal space that prevents complete maneuverability of the trocar. Although the LA provides excellent anesthesia to the immediate area of entry, it has no effect on the ribs which, if they are forcefully pushed up or down by the movement of the thoracoscope, may induce pain. Another scenario requiring a second port of entry is when large adhesions are present that require cutting or circumnavigation. This is particularly important if the adhesions are vascularized and require coagulation to safely separate them (Fig. 7.8).
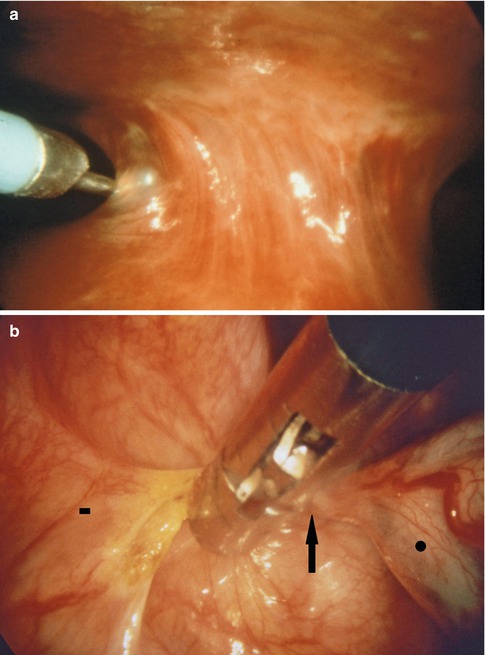

Fig. 7.8
Large adhesions can be severed using an electrocautery loop (a) (in this case, on the left side, through a second point of entry) or coagulating forceps (b). This is particularly important if the adhesions are highly vascularized requiring coagulation to safely separate them. Vascularized adhesion (arrow), parietal pleural side (square), lung (circle)
If a thoracoscopy is performed, it is paramount that a full inspection of the cavity is undertaken from the apex to the diaphragm. If this is not feasible through a single port, then a second access point should be created. As previously discussed, if electrocautery is needed for control of hemorrhage, then the physician must be able to create a second port of entry rapidly. This second port can be placed rapidly under direct vision. The chosen site is viewed through the primary port with the optic while the assistant depresses on the chest wall at the second site of entry. The external compression can be easily visualized on the internal chest wall (Fig. 7.9a, b). The trocar can then be placed rapidly under direct vision as described later in this chapter.
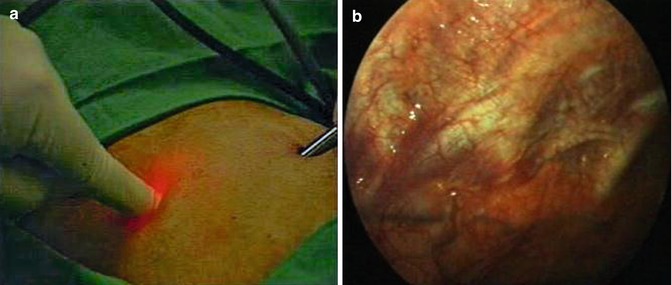

Fig. 7.9
A 5-mm trocar can be placed rapidly under direct vision through a second point of entry. (a) The chosen site is viewed through the primary port with the optic, while the assistant depresses on the chest wall at the second site of entry. (b) The external compression can be easily visualized on the internal chest wall
7.3.2 Alternative Equipment
Thoracoscopy can be performed using alternative equipment apart from that described above. Indeed a flexible bronchoscope has been used for inspection of the pleural cavity! Recently, a dedicated semirigid/flexible thoracoscope has been developed (Chap. 26). There are advantages and disadvantages associated with this equipment. The rigid thoracoscope provides excellent vision, large biopsy samples via a single port of entry, ease of biopsy from harder lesions, and easy orientation inside the pleural cavity. However, physicians who are skilled with the flexible bronchoscope may feel more comfortable with the semirigid thoracoscope. The flexible equipment allows lateral vision or retro visualization (this would require a separate oblique view telescope if using rigid instruments) (Ishida et al. 2011).
However, when compared with rigid instruments, the control of the working end of the flexible thoracoscope is limited due to its flexibility. In addition, the biopsy size is small which in turn limits its diagnostic yield, especially in the setting of malignant mesothelioma. Finally, the initial access to the pleural space still requires placement of a trocar which is the same diameter as that used for rigid equipment. Therefore there is no reduction in the discomfort experienced by the patient during the procedure.
Therefore, despite the obvious attraction of the semirigid thoracoscope to respiratory physicians – because of its similarity to the bronchoscope – it is limited by the inherent disadvantages of flexible instruments including the fragility of the instruments, sterilization requirements, high maintenance costs, and small size of the biopsies. We believe that there are no clear advantages of the flexible thoracoscope over the rigid equipment.
Smaller equipment, so-called minithoracoscopy, has also been developed as an alternative for diagnostic thoracoscopy under local anesthesia. This consists of rigid equipment using either a 2- or 3-mm thoracoscope. Tassi et al. obtained a diagnostic yield of 93 % using a 3-mm thoracoscope. In a paper comparing 2-mm, 3-mm, and standard thoracoscopes, the authors obtained a diagnostic yield of 40, 100, and 100 % for the 2-, 3-, and 7-mm equipment, respectively (Tassi et al. 2011).
One of the disadvantages of the mini equipment is that it is necessary to create a second port of entry in order to obtain biopsies. This compares to the standard equipment where all procedures can be performed through a single port of entry.
7.4 Technique
7.4.1 Preparation of the Patient
Although thoracoscopy is a safe and relatively simple procedure – if the performing physician is well trained and familiar with the endoscopic anatomy of the thorax – a few simple rules, which apply to all endoscopic procedures, should be followed carefully.
7.4.1.1 Preoperative Discussion with the Patient
Although this is a basic requirement for informed consent, it is especially important when the procedure is performed under local anesthesia as the patient will be more confident during the intervention if he or she knows the details of the procedure in advance.
7.4.1.2 Preoperative Assessment
The patient’s medical status should be optimized prior to the thoracoscopy. The procedure should be deferred for subjects with uncontrolled cough. This makes the procedure very difficult for both the patient and physician alike and results in complications (subcutaneous emphysema). Great care should be taken with patients with a poor performance status, hypoproteinemia, or diffuse neoplastic infiltration of the chest wall. Ultimately physicians should try to balance the benefits with the risks of the procedure. As with all interventions the procedure should not be performed unless there is a definite benefit to the patient.
7.4.1.3 Preoperative Investigations
All patients undergoing thoracoscopy should have a preoperative ECG. Patients with unstable angina or a history of a recent myocardial infarction should be rejected for thoracoscopy. Coagulation and blood gas analysis should be performed preoperatively. Extreme care should be taken with patients with hypercapnia especially if the PaCO2 is greater than 55 mmHg. Hematological abnormalities should be corrected, if possible, prior to the intervention. Patients with pancytopenia or coagulation disorders are at an increased risk of complications, and indeed thoracoscopy is contraindicated if the platelet levels are below 60,000/mm3. The INR should be less than 2.0. The use of aspirin may prolong bleeding time, but is not an absolute contraindication to biopsy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree