Chapter 35 Surgical Therapy for Aortic Dissection
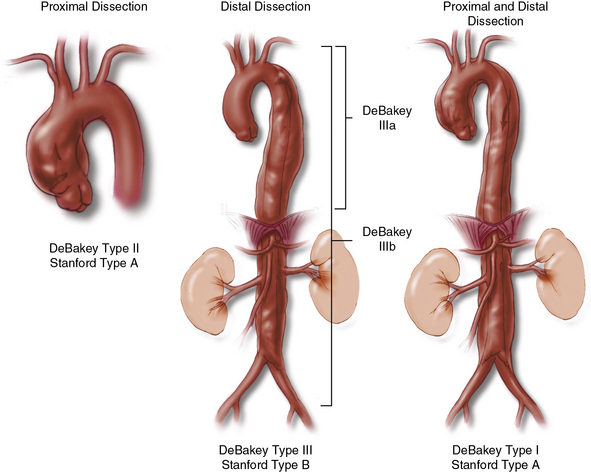
The treatment of aortic dissections remains technically challenging to surgeons. Patients can present with a wide range of anatomical and physiological derangements. Surgical decisions are made on the basis of three primary considerations: anatomical location of the dissection, time since the onset of dissection, and resulting complications of dissection. The DeBakey and Stanford classifications define dissections according to their anatomical location; both systems place great importance on the involvement of the ascending aorta1 (Fig. 35-1). DeBakey type I dissection initiates in the ascending aorta and extends varying distances into the thoracoabdominal aorta, often reaching the aortic bifurcation. Type II dissection is confined to the ascending aorta. Type III dissection initiates in the descending thoracic aorta and extends variable distances into the thoracoabdominal aorta. Timing of the operation is important because surgical repair becomes safer as the dissection becomes older and the aorta less fragile. Risks posed by tissue fragility must be weighed against the competing risk of acute complications, which include rupture, heart failure, and malperfusion. Although arbitrary, dissection is considered acute within the first 14 days after the initial tear in the aortic wall. After 14 days, the dissection is described as chronic.
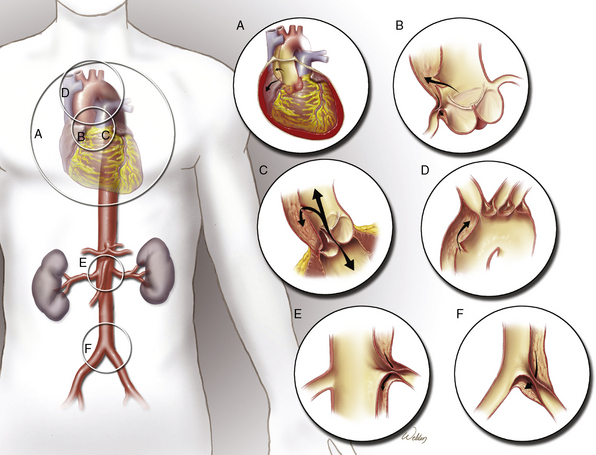
Additionally, aortic dissections can produce a wide variety of life-threatening complications that may mandate emergent surgical repair or correction. Aortic rupture can occur anywhere along the dissected aorta. Lethal proximal aortic complications include pericardial tamponade, acute aortic valve regurgitation, and myocardial infarction (MI) from coronary artery malperfusion. In subsequent aortic segments, malperfusion of branch vessels can cause stroke, paraplegia, mesenteric ischemia, renal failure, and limb-threatening ischemia (Fig. 35-2). The potential for these acute complications, combined with severe physiological derangement and extreme tissue fragility, make aortic dissection one of the most challenging conditions faced by cardiovascular surgeons. These considerations are the foundations of operative indications and strategies for aortic dissection. Surgical strategies for treating proximal aortic dissections involving the ascending aorta and transverse aortic arch differ distinctly from strategies for treating distal aortic dissections involving the descending thoracic and thoracoabdominal aorta; therefore, the proximal and distal aortic segments will be discussed independently.
Acute Proximal Dissection
Without treatment, nearly half of patients with acute proximal aortic dissection die within 48 hours.2 Once the diagnosis is suspected, aggressive pharmacological treatment is initiated immediately, and the focus can then shift to confirming the diagnosis and assessing treatment options (see Chapter 34).
Indications for Operation
Proximal aortic repairs performed in the chronic phase uniformly have better outcomes than those performed in the acute phase. Unfortunately, the high risk associated with early operation is outweighed by the even higher risk of a fatal complication (e.g., aortic rupture) during medical management. Therefore, the presence of an acute proximal aortic dissection has traditionally been considered an absolute indication for emergency surgical repair. Many authors continue to advocate this approach.3,4 Although controversial, delayed operative management of acute proximal aortic dissection has been proposed in specific clinical scenarios: in elderly patients; in patients with severe malperfusion; when dissection occurs after previous cardiac operation; and to enable transport to a specialized center.
Elderly patients
Emergent repair of proximal aortic dissection in patients with advanced age remains controversial. In recent literature, operative mortalities of nearly 50% have been reported for octogenarian patients.5,6 Neri et al. concluded that surgical treatment is not warranted in the elderly because “it does not reverse the unfavorable prognosis of the disease.”7 A demographic study that used a Taiwanese national registry identified a significant trend toward nonoperative management of acute aortic dissections (AAD) with increasing age.8 However, elderly patients who survived operative treatment had better long-term survival than patients who were treated medically.
Clearly, acute proximal aortic dissection in the elderly is a high-risk situation. Surgical results of institutions and communities have to be considered to optimize best outcomes.9,10 Aggressive intraoperative strategies, such as total arch replacement and root replacement, should be weighed against the mortality risk associated with prolonged operations. In patients whose limited physiological reserve makes them poor candidates for emergency aortic repair, delayed management with initial medical optimization followed by elective surgery may be a reasonable alternative.
Severe malperfusion
Branch-vessel obstruction due to dissection creates a spectrum of malperfusion that ranges from mild (e.g., diminished pulse in an extremity) to severe (e.g., bowel infarction). In most cases of mild to moderate malperfusion, surgical repair of the proximal aorta redirects flow into the true lumen and restores adequate peripheral blood flow; however, patients in whom ischemia has caused severe end-organ dysfunction are unlikely to benefit from immediate ascending aortic repair.11 Stroke with resulting coma and bowel infarction with peritonitis remain ominous conditions after type I aortic dissection. Deeb et al. reported that eight of nine patients who underwent early proximal aortic repair in the setting of severe malperfusion (as defined in Box 35-1) died before discharge from the hospital. All deaths were attributed to irreversible ischemic organ damage and severe reperfusion injury after cardiopulmonary bypass (CPB). On the basis of these results, these surgeons initiated a policy of delayed surgical treatment in patients with severe malperfusion. This strategy consisted of aggressive pharmacological treatment to reduce dP/dt (rate of rise of left ventricular [LV] pressure), confirmatory arteriography, percutaneous fenestration or stenting (if needed) to restore flow to compromised branch vessels, and elective operation after complete recovery from malperfusion. Of the 20 patients treated with this strategy, 17 underwent delayed operation an average of 20 days after presentation.12 To reduce selection bias, the authors’ analysis also included the three patients who died without operation (one from rupture, two from reperfusion injury) and the two patients who died after delayed surgery. The overall survival for these patients treated without immediate operation (15/20, 75%) was significantly better than the dismal survival obtained with a strategy of immediate surgery. Fabre et al. have also advocated percutaneous intervention before operation in patients with severe ischemic sequelae.13
![]() Box 35-1 Definitions of Severe Malperfusion
Box 35-1 Definitions of Severe Malperfusion
Adapted from Deeb GM, Williams DM, Bolling SF, et al: Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg 64:1669–1675, 1997.
Dissection after prior cardiac operations
Delayed management with elective operation has been proposed for patients who have had cardiac surgery in the remote past. Presence of prosthetic aortic valves, aortic suture lines, coronary grafts, and adhesions due to scarring and fibrosis around the aortic wall are considered protective. They can potentially prevent rupture, protect from valvular dehiscence, and prevent coronary malperfusion, the lethal complications of proximal aortic dissection. In one study by the International Registry of Acute Aortic Dissection (IRAD) investigators, patients with acute proximal dissection and a history of previous cardiac operations were less likely to present with chest pain and cardiac tamponade than those without a history of previous cardiac operations.14 Hassan et al. explored the strategy of initial medical management in ten patients with ascending aortic dissection in the setting of prior cardiac surgery. Duration from prior cardiac surgery to dissection ranged from 2 months to 20 years. Medical therapy was successful in eight patients (80%), and all patients were discharged after the initial hospitalization. The two deaths in the series occurred at 4 months and 2 years after the dissection.15
It must be emphasized that the reduced risk of rupture does not apply to dissections that occur during the initial 3 weeks after cardiac surgery.16 In fact, acute dissection during the early postoperative period carries a high risk of rupture and tamponade; these patients should undergo early operation.17 Estrera et al. noted higher operative mortality (31% vs. 14%) and higher stroke rates (10% vs. 3%) in patients undergoing early operative repair for acute proximal dissection with prior cardiac surgery than in patients without prior cardiac surgery.18 In their series, patients with prior cardiac surgery presented with similar incidences of tamponade and malperfusion symptoms as patients without prior cardiac surgery, although the series included patients with acute dissections occurring as early as 3 days after cardiac surgery.
Transport to specialized centers
Patients with proximal aortic dissections frequently require transport to centers where cardiac surgery can be performed. Even in centers where cardiac surgery is available, transfer to high-volume centers can be justified in hemodynamically stable patients, and there is evidence of improved outcomes in patients transferred to specialized centers.19–21 Before proceeding with transport, the patient’s condition must be optimized. Aggressive pharmacological management should be initiated and metabolic disturbances corrected. Reliable delivery and titration of vasoactive medications during transport can be facilitated by central venous and arterial catheters, respectively. Inotropes and diuretics can be administered to patients with low cardiac output and acute ventricular distention due to aortic valvular insufficiency and volume overload. If patients with pericardial tamponade must be transferred, a pericardial drain should be placed to allow intermittent drainage during transport.22 Whenever possible, patients with limb-threatening ischemia should undergo revascularization—usually via femoral-to-femoral artery bypass—before transport to minimize the severe metabolic derangements that result from prolonged limb ischemia and improve chances of survival after aortic repair.12
Standardized treatment protocols have been developed to optimize the hemodynamic management of patients with AAD during transport. Implementation of the protocol developed by the Stanford Health Care Life Flight program decreased the number of patients who arrived at the receiving center with inadequate blood pressure control.23 Results from a German study show that transporting patients with proximal aortic dissections by helicopter is no better than emergency ground transport with regard to survival benefit. Air transport did allow coverage of areas more than twice the distance, but at eight times the cost.24
Surgical Repair
Cardiopulmonary bypass
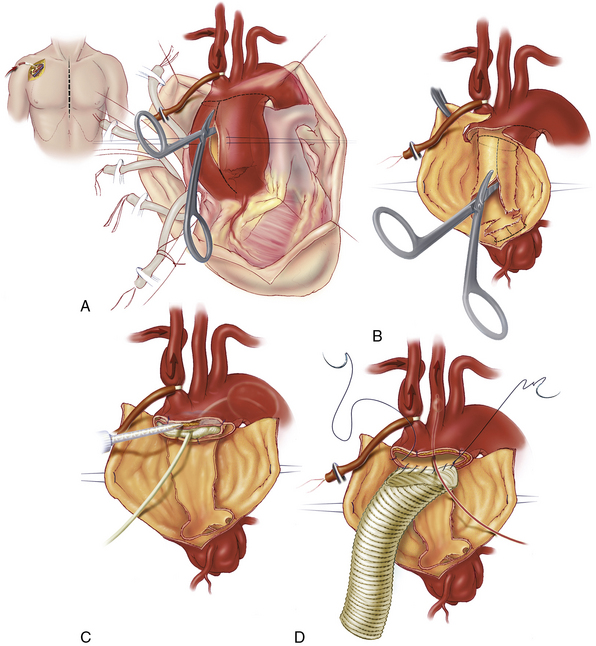
Anterior exposure by median sternotomy provides standard access to the heart and proximal aorta. Most surgeons perform proximal aortic dissection repairs during a period of hypothermic circulatory arrest.3,11,25–27 This strategy allows an “open distal anastomosis” with direct inspection of the entire arch and avoids creating additional tears that can result from placing a clamp across the fragile aorta. Peripheral options in cannulation for arterial inflow during mechanical circulation include the femoral artery and axillary artery. Many groups currently advocate axillary access by either direct cannulation or graft conduit. The axillary site usually allows perfusion of true lumen and simplifies antegrade cerebral perfusion.28 Previously, femoral cannulation was the most common site of arterial inflow in acute dissections. One advantage is rapid access in emergent situations, although malperfusion and retrograde atheroembolization can occur. Central aortic perfusion, either by direct ascending aortic cannulation29 or advancement of the cannula into the ascending aorta via the LV apex, is a feasible alternative. Venous drainage is typically achieved with the use of a dual-staged cannula placed in the inferior vena cava (IVC) via the right atrium.
Two methods of cerebral protection are hypothermia and cerebral perfusion. Hypothermia alone decreases metabolic activity to allow circulatory arrest, but surgeons must be aware of time limitations to ensure good neurological outcomes.30,31 Although use of retrograde cerebral perfusion has declined over the past decade, the technique is still used in some centers. Retrograde cerebral perfusion delivers cold oxygenated blood from the pump into a cannula placed in the superior vena cava.26,32 The initial hope was that the retrograde delivery of blood would provide oxygen to the brain. Unfortunately, accumulating evidence suggests that this technique does not provide cerebral oxygenation.33–35 The benefits of this technique include maintenance of cerebral hypothermia and retrograde flushing of air and debris.
We and others currently use selective antegrade cerebral perfusion as a standard adjunct in proximal dissection repairs.36,37 Direct cannulation of the innominate and left carotid arteries can be performed using flexible balloon catheters. However, right axillary cannulation for CPB can provide direct flow into the right carotid28,38,39 (Fig. 35-3). Traditionally, circulatory arrest was initiated with deep hypothermia at 18°C, but this level of hypothermia has negative implications in CPB duration and degree of coagulopathy. Recent experience supports the safe use of moderate hypothermia during circulatory arrest.40 Our current target temperature on CPB is 24°C. Once the target temperature is reached, CPB flows are decreased to 1 to 1.5 L/min. A snare is used to occlude the innominate artery, thereby initiating circulatory arrest to the body and antegrade right cerebral perfusion. With the aorta open, we selectively use left carotid perfusion by a separate balloon catheter on the basis of the anticipated length of circulatory arrest and near-infrared spectroscopy (NIRS) cerebral monitoring. Electroencephalography (EEG) is useful during deep hypothermia when cerebral electrical silence is desired. However, with moderate hypothermia, EEG silence is usually not achieved.
Distal aortic considerations
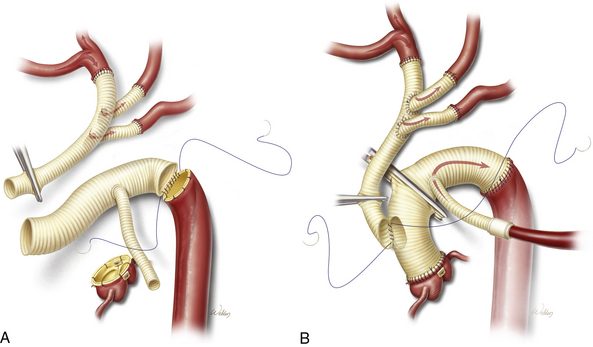
With the ascending aorta opened, the transverse aortic arch can be carefully inspected, and a decision can be made regarding the extent of aortic arch resection (Box 35-2). At the least, most patients require graft replacement of the segment of the ascending aorta between the sinotubular junction and the origin of the innominate artery. In the setting of emergent operation for acute dissection, increasingly aggressive repairs of the aortic arch are associated with increasing early morbidity and mortality.41 Therefore, the repair is generally only extended into the arch if the arch is aneurysmal or if the primary tear is located within the arch. When only the proximal portion of the arch is involved in the disease process, a beveled graft replacement of the lesser curvature is performed (see Fig. 35-3). This open distal hemi-arch replacement remains the most common scenario. Total arch replacement (Fig. 35-4) is performed only if the primary tear is located in the arch or if the entire arch is aneurysmal. If malperfusion was an issue preoperatively owing to true lumen compression in the descending thoracic aorta, patency of the true lumen can be assisted by open placement of an endovascular stent-graft in the descending thoracic aorta.
Ascending and hemi-arch replacement
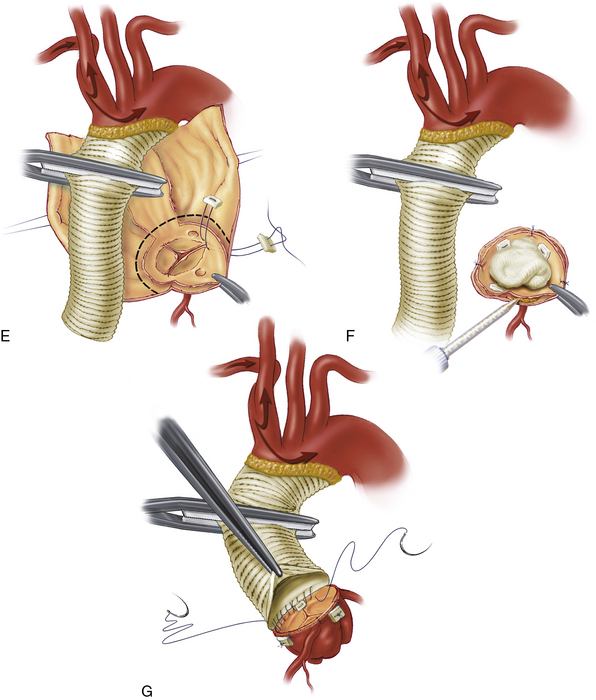
The dissecting membrane that separates the true and false lumens is excised to the distal aortic cuff (see Fig. 35-3B). The distal aortic cuff is prepared by tacking the inner and outer walls together and using surgical adhesive to obliterate the false lumen and strengthen the tissue11,25 (see Fig. 35-3C). A Foley catheter balloon carefully inflated at the distal aortic arch can be helpful in preventing surgical adhesive from migrating distally in the false lumen. A polyester tube graft is sutured to the distal aortic cuff (see Fig. 35-3D). With the false lumen obliterated at the distal aortic cuff, the anastomosis between the graft and the aorta is constructed to a single true lumen; this often alleviates mild distal malperfusion problems that were present preoperatively. We routinely reinforce the distal anastomosis with a second suture line or interrupted pledgets. The graft is de-aired and clamped, and full CPB is resumed with the release of the innominate snare. Rewarming is initiated, and the proximal portion of the repair is started (see Fig. 35-3E).
Total aortic arch replacement
Extensive aneurysms involving the entire arch usually require total arch replacement. Primary tears affecting the greater curvature or any of the brachiocephalic branch vessels should be resected. Distal anastomosis is created beyond the primary tear at the transverse arch or at the proximal descending thoracic aorta, using a tube graft. Our preference currently is for reattachment of the brachiocephalic vessels individually, using a trifurcated or bifurcated graft42 (see Fig. 35-4). The single outflow to the brachiocephalic branches is anastomosed to the ascending aortic graft. In the most extreme cases, the aneurysm extends past the arch and into the descending thoracic aorta. This can be managed using Borst’s elephant trunk technique for total arch replacement.43,44 The distal anastomosis is constructed so that a portion of the graft is left suspended within the true lumen of the proximal descending thoracic aorta. In addition to directing flow into the true lumen, this “trunk” can be used to assist repair of the descending thoracic aorta during a subsequent operation.
Antegrade descending thoracic stent-grafts
Even with aggressive resection of the primary intimal tear and elimination of the false lumen at the distal aortic anatomosis, the false lumen often persists at the level of the descending thoracic aorta and beyond. The distal false lumen presents two considerations. First, presence of a false lumen after proximal aortic dissection continues to be a significant risk factor for late aneurysm formation, need for reoperation, and death.45–47 Second, and more important in the acute setting, true lumen compression in the descending thoracic aorta can cause malperfusion in the mesenteric vessels, renal vessels, and lower extremities. Concurrent endovascular stent-graft deployment in the descending thoracic aorta with either standard ascending or hemi-arch reconstruction or in an extended total arch reconstruction in a “frozen elephant trunk” are options other investigators are exploring.48–50
Proximal aortic considerations
Presence of preexisting annuloaortic ectasia/aortic root aneurysm or connective tissue disorder, the degree to which the dissection flap extends into the root, and the degree of aortic valve distortion are some of the factors for consideration when evaluating a proximal dissection for repair. Potential repairs addressing the aortic valve are listed in Box 35-3.
Supracommissural anastomosis with aortic valve repair
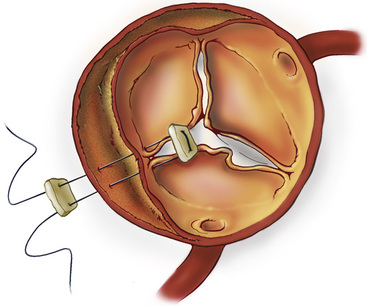
In the absence of intrinsic aortic root pathology and significant aortic valve distortion, the root can be repaired. The majority of these patients have separation of one or more commissures from the outer aortic wall; the resulting valve regurgitation can be corrected by resuspending the commissures into their normal position51 (Fig. 35-5). Many surgeons use surgical adhesive within the false channel to strengthen this aortic root reconstruction.12,25,52 The proximal aortic cuff is prepared with tacking sutures and surgical adhesive (see Fig. 35-3F) before performing the proximal aortic anastomosis (see Fig. 35-3G). If there is mild to moderate annular dilation, a commissural plication annuloplasty helps restore and maintain effective leaflet coaptation. Once the root and valve repairs are complete, the proximal aortic anastomosis is completed at the supracommissural position.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree