Chapter 21 Reconstructive Surgery for Peripheral Artery Disease
The clinical manifestations and complications of atherosclerosis are the most common therapeutic challenge encountered by vascular surgeons. The tendency for lesions to develop at specific anatomical sites and follow recognizable patterns of progression was appreciated as long ago as the late 1700 s by the extraordinary British anatomist and surgeon John Hunter. Considered one of the forefathers of vascular surgery, his dissections of atherosclerotic aortic bifurcations remain on view at the Hunterian museum in London and presage the disease process Leriche would give a name to 150 years later.1
The modern era of surgical reconstruction for complex atherosclerotic occlusive disease began in earnest in 1947 when the Portuguese surgeon J. Cid dos Santos successfully endarterectomized a heavily diseased common femoral artery (CFA).2 Four years later in San Francisco, Wylie et al. extended this new technique to the aortoiliac level.3 At the same time, and building on the pioneering work of Alexis Carrel,4 Kunlin5 would report the first long-segment vein bypass in the lower extremity. It would be another 10 years before synthetic grafts were being regularly used for aortic bypass grafting and the first efforts to extend vein grafting to the tibial level were described by McCaughan.6 Tremendous advances in our understanding of atherosclerosis biology and ability to percutaneously treat arterial occlusive disease have dramatically affected treatment algorithms for arterial insufficiency in recent years. This chapter will review the current role for surgical management of aortoiliac and infrainguinal arterial occlusive disease.
Aortoiliac Occlusive Disease
Chronic obliterative atherosclerosis of the distal aorta and iliac arteries commonly manifests as symptomatic arterial insufficiency of the lower extremities. Disease in this location is seen often in combination with occlusive disease of the femoropopliteal arteries, producing a range of symptoms from mild claudication to more severe levels of tissue loss and critical ischemia. Patients with hemodynamic impairment limited to the aortoiliac system may have intermittent claudication of the calf muscles alone or involvement of the thigh, hip, and/or buttocks. If disease distribution also targets the hypogastric vessels, patients may additionally suffer from difficulty in achieving and maintaining an erection due to inadequate perfusion of the internal pudendal arteries. The equivalent impact of impaired pelvic perfusion in women remains poorly understood but has attracted investigative attention.7 A well-characterized constellation of symptoms and signs known as Leriche’s syndrome, associated with aortoiliac occlusive disease in men, includes thigh, hip, or buttock claudication, leg muscle atrophy, impotence, and reduced femoral pulses.8
Although atherosclerotic disease limited to the aortoiliac region commonly gives rise to claudication of varying degrees, it is rarely associated with lower-extremity ischemic rest pain or ischemic tissue loss. This is largely the result of adequate collateralization around the point of obstruction via lumbar, sacral, and circumflex iliac vessels that serves to reconstitute the infrainguinal system with enough well-perfused arterial blood to ensure sufficient resting tissue perfusion (Fig. 21-1). A well-recognized exception to this general observation arises in the situation of embolic disease. Blue toe syndrome represents a situation where atherosclerotic debris breaks free from an aortic or iliac plaque and embolizes to distal vessels.9,10 Wire manipulation during coronary or peripheral angiographic procedures and cross-clamping across a calcific aortic plaque during cardiac surgery are common sources of such emboli. The terminal target of the microembolic particles, be they cholesterol crystals, calcified plaque, thrombus, or platelet aggregates, is typically the small vessels of the toes.
If aortoiliac occlusive disease is found in combination with femoropopliteal occlusive disease, ischemic rest pain or even more severe perfusion impairment leading to ischemic tissue loss or gangrene is not uncommon.11 Such progressive disease affecting multiple levels of the peripheral vasculature tree is most frequently encountered in the elderly. Approximately a third of patients operated on for symptomatic aortoiliac occlusive disease have orificial profunda femoris occlusive disease, and more than 40% have superficial femoral artery (SFA) occlusions. Aortoiliac disease typically begins at the distal aorta and common iliac artery (CIA) origins, and slowly progresses proximally and distally over time.12 This progression is quite variable but may ultimately extend to the level of the renal arteries or result in total aortic occlusion.
A particularly virulent form of atherosclerotic arterial disease is often found in young women smokers.13 Radiographic imaging in this subset of patients typically reveals atretic narrowed vasculature with diffusely calcific atherosclerotic changes. Frequently, a focal stenosis is found posteriorly near the aortic bifurcation. This particular distribution of disease and the characteristic patient profile have been referred to as small aorta syndrome14 (Fig. 21-2). Such patients invariably have an extensive smoking history, with or without other typical factors for atherosclerosis. Given the diminutive size of the aorta and iliac vessels, the durability of endovascular intervention is generally inferior in these patients, particularly in the face of continued cigarette use.
The diagnosis of aortoiliac occlusive disease is generally made based on patient symptomatology, physical examination, and noninvasive tests such as segmental pressure measurements and pulse volume recordings (PVRs) (see Chapters 11 and 12). Following diagnosis of aortoiliac disease and the decision to pursue intervention, further imaging is warranted. In many centers, magnetic resonance angiography (MRA) and computed tomographic angiography (CTA) have supplanted contrast angiography as the initial imaging studies of choice. Advances have solved many of the technical limitations of earlier studies, and reliable roadmaps to guide operative planning are now reproducibly obtainable. Both MRA and CTA allow for a comprehensive view of the vascular tree with three-dimensional (3D) reconstructions (see Chapters 13 and 14). Computed tomographic angiography has the additional benefit of evaluating vessel morphology beyond the flow lumen, and allows for appreciation of degree of vessel calcification as well as anatomical localization based on surrounding structures. Angiographic findings of CTA compare favorably to standard digital subtraction angiography (DSA).15 Should a lesion amenable to percutaneous therapy be identified on MRA or CTA, formal angiography is then pursued. In cases in which a good-quality roadmap is obtained with MRA or CTA, and the clinical situation or anatomical pattern is unfavorable to a percutaneous approach, surgery can in most instances be planned directly, obviating the need for traditional subtraction angiography.16
In the minority of cases necessitating DSA for preoperative planning, a retrograde femoral approach is typically used, whereas the transbrachial approach serves as a useful alternative in patients with particularly challenging anatomy.17 Additional lateral and oblique views of the abdominal aorta are advised if concomitant mesenteric or renal occlusive disease is present, and multiple projections of the iliac and femoral bifurcations are essential in clarifying the extent of disease in these regions (see Chapter 15). Finally, full runoff views of the lower extremities are needed to assess the presence or absence of femoropopliteal or crural disease. In ambiguous cases, pullback pressure measurements, both before and after administration of a systemic vasodilator such as papaverine or nitroglycerine, or application of a tourniquet to induce reactive hyperemia can be useful in documenting the hemodynamic significance of a particular stenotic zone.18
Management
Risk factor modification remains a cornerstone of management of aortoiliac occlusive disease (see Chapter 19). Smoking cessation, blood pressure control, and aggressive efforts at cholesterol lowering should be addressed with every patient with atherosclerotic disease. Strong evidence exists supporting the benefit of a structured walking program19 in increasing walking distance of patients with claudication. The benefit of walking outside of a structured regimen with close follow-up is more debatable.20 Medical management with cilostazol has benefit in a subset of patients and is a reasonable first-line approach to improving claudication symptoms.21
A considerable change in the management approach to claudication has taken place in recent years. Patients suffering from disabling claudication, rest pain, or ischemia-related tissue loss warrant serious consideration for arteriography and either percutaneous or surgical intervention. Previously, however, such aggressive treatment would have been considered inappropriate for claudication that was not clearly disabling. As percutaneous treatment has become increasingly safer and more effective, however, and its application spread to increasingly more arterial beds, indications for endovascular revascularization have correspondingly increased (see Chapter 20). Such a sea change in the overall management approach to aortoiliac disease has had a dramatic impact on the numbers of patients now proceeding to open surgery. Just as escalating use of renal angioplasty and stenting for renal occlusive disease has led to a considerable drop in open surgical renal artery reconstructions, the rising popularity and success of aortic and iliac balloon angioplasty and stenting as first-line therapy has noticeably reduced the volume of aortoiliac reconstructive procedures performed in this country.
When medical therapy or percutaneous treatment has proven to be inadequate or is technically inadvisable, open surgical revascularization remains indicated for those patients with aortoiliac disease and disabling claudication, ischemic rest pain, and ischemic ulceration or gangrene. Patients with nighttime foot rest pain or tissue loss usually have multisegment disease, and the decision whether to perform both supra- and infrainguinal revascularization procedures or to perform only an inflow procedure is guided by severity of the ischemia.11,22–24 In general, patients presenting with significant tissue loss or gangrene are much more likely to require simultaneous or staged inflow and outflow procedures.
Endarterectomy
Aortic endarterectomy was commonly performed in the early era of aortoiliac reconstruction.25,26 Although it is particularly suited to localized disease limited to the distal aorta or proximal iliac arteries, it has proven to be less reliable for disease involving the entire infrarenal aorta and extending into the external iliac arteries (EIAs).27,28 The obvious benefit of endarterectomy is elimination of the need for a prosthetic graft, removing the possibility of myriad late graft-related complications. Long-term patency of limited endarterectomy is excellent and on par with bypass procedures.29 However, the number of patients suitable for this reconstructive approach is small and continues to diminish in the era of endoluminal reconstruction. Experience with endarterectomy during one’s training or early surgical career is another important factor influencing the choice of therapy offered because significant technical expertise is required, and many surgeons in the current era have limited familiarity with this approach.
Aortobifemoral Bypass
Aortobifemoral bypass remains the mainstay of operative treatment for aortoiliac occlusive disease. During the last 20 years, the procedure has supplanted both aortic endarterectomy and aortoiliac bypass procedures. In the latter case, this change was largely driven by recognition of subsequent graft failure due to progression of native iliac arterial disease.27,30 Early experience with aortobifemoral grafting in the 1970s was associated with a 5% to 8% 30-day operative mortality rate.28,29,31,32 Over recent decades, mortality rates of 1% have been reported, on par with those of elective abdominal aortic aneurysm repair.33,34
Typically, half of patients proceeding to surgery for aortoiliac occlusive disease will have significant coronary artery disease, (CAD) even more will have hypertension, and almost 80% will be current or earlier cigarette smokers.33 The reduced mortality and morbidity seen in recent years are in large part due to advances in management of concomitant coronary disease. Specifically, the importance and benefit of better preoperative identification of patients in need of initial coronary revascularization, awareness of the benefit of waiting an interval period following coronary stenting before proceeding with major noncoronary vascular surgery, improved perioperative pharmacological management of patients with impaired myocardium, and more focused efforts to tailor operative and postoperative fluid administration to the individual patient’s myocardial reserve are all well recognized.35,36 General advances in postoperative intensive care unit management, including pulmonary care, infection control, and blood product utilization, have further contributed to the progress seen.
Current early patency rates for aortobifemoral bypass grafting are excellent, approaching 100% in many reporting institutions. Five-year patency rates are greater than 80%,29,31–33,37 whereas 10-year rates are near 75%.29 There are multiple reasons for the improved patency. The current graft material used by most surgeons for aortoiliac reconstruction is a knitted Dacron prosthesis with enhanced hemostatic properties; it tends to have a more stable pseudointima than earlier-used woven grafts.38,39 More attention is paid to avoiding graft redundancy and ensuring a good size match between the graft and the recipient vessels. Grafts are more routinely extended beyond the iliac level to the femoral vessels, which not only improves exposure and makes for a technically easier distal anastomosis but is also associated with less graft thrombosis from unanticipated progression of atherosclerotic disease in the external iliac vessels.30 With meticulous skin preparation, close attention to draping, careful surgical technique, and judicious use of a short course of intravenous antibiotic therapy, the feared higher rate of graft infection from placing the distal dissection at the groins has not materialized.40 An exception to this general practice is recommended in certain circumstances, however. For example, patients with hostile groin creases from prior surgery or radiation therapy, or obese diabetic patients with an intertriginous rash at the inguinal crease, will all likely be better served by performing the distal anastomosis at the external iliac level if their anatomy for such is suitable.
Increased awareness of the critical role played by the deep femoral artery (DFA) in preserving long-term patency of aortobifemoral grafts29,32,41,42 has also undoubtedly contributed to the better results seen. This awareness parallels a better overall appreciation for the importance of establishing adequate outflow at the femoral level in achieving higher early and late graft patency rates and sustained symptom relief. The true impact of concomitant SFA disease is unclear from the literature. Some reports have indicated similar patency rates between those patients with and without SFA occlusion,22,23 whereas others have suggested late patency rates are reduced in this setting.40,43 What has definitely been shown is the benefit of a profundaplasty in the presence of significant superficial and profunda femoral occlusive disease.44,45 Some authors have even recommended that a profundaplasty should be carried out in every case of superficial artery occlusion, even in the absence of orificial profunda disease, arguing that a “functional” obstruction on the order of 50% stenosis is present in these patients.46 Although this position has not been universally adopted, it is now common practice to extend the hood of the distal anastomosis over the origin of the profunda femoral artery to enhance the graft outflow, especially in situations in which the SFA is occluded or severely diseased. In the presence of significant common femoral or profunda femoral origin plaque, an extensive endarterectomy and/or profundaplasty is indicated (Fig. 21-3). In these circumstances, it is preferable to close the endarterectomized recipient bed with a vein, bovine pericardial patch, or Dacron patch onto which the distal anastomosis can then be attached, rather than creating a long femoris patch with the graft limb.41
Several technical considerations related to aortobifemoral bypass grafting are the subject of considerable and passionate debate. The first involves the manner of the proximal anastomotic creation. Advocates of an end-to-end configuration claim that it facilitates a more comprehensive thromboendarterectomy of the proximal stump and allows for a direct, more inline flow pattern with less turbulence and more favorable flow characteristics.47 Obviation of competitive flow through the excluded iliac vessels with this approach is likely more of theoretical rather than real benefit. Certainly, with concomitant aneurysmal disease or complete aortic occlusion extending up to the level of the renal arteries, end-to-end grafting is indicated. Creation of an end-to-side anastomosis can at times be technically challenging in a heavily diseased aorta partially occluded by a side-biting clamp. A lower rate of proximal suture line pseudoaneurysms and better long-term patency rates have been found in some series.48 Stapling or oversewing of the distal aorta with the end-to-end technique minimizes the immediate risk of clamp-induced emboli to the lower extremities following release of the distal clamp. Finally, those in favor of this approach claim that ability to more effectively close the retroperitoneum, particularly after resection of a short segment of the infrarenal aorta, results in lower rates of late graft infection and aortoenteric fistulae, although there is no direct evidence to support this assertion.
There are certain circumstances when an end-to-side proximal anastomotic configuration is advantageous. The most common indication involves those patients with occluded external iliac arteries, in whom interruption of forward aortic flow may result in loss of perfusion to an important hypogastric or inferior mesenteric artery (IMA) and consequent significant pelvic ischemia. Colon ischemia (1%-2%),49 or even more rarely, paraplegia secondary to cauda equina syndrome (< 1%),50 are additional complications that can be avoided by an end-to-side configuration. Although advocated by some,51 routine preservation of a patent IMA is not universally practiced.
Operative Management
The proximal reconstruction is performed via a midline laparotomy. In general, aortic dissection is limited to the region between the renal arteries and the inferior mesenteric artery. This allows avoidance of extensive dissection anterior to the aortic bifurcation, where the autonomic nerve plexus regulating erection and ejaculation in men sweeps over the aorta. An intriguing recent survey indicated no significant differences in the rate of sexual dysfunction with open compared with endovascular repair of abdominal aortic aneurysms, suggesting the effects of aortic dissection in this area are perhaps less important than typically believed.52
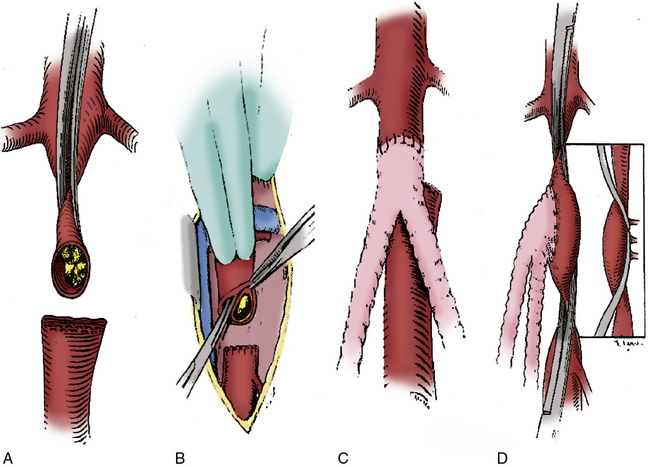
In situations where significant aortic calcification extends up to the level of the renal arteries, it may be necessary to continue aortic dissection to the suprarenal or even supraceliac level to allow for safe proximal clamp placement. Alternatively, proximal control may be obtained by intraluminal balloon deployment. If end-to-side repair is planned, circumferential dissection of the aortic segment to be clamped is recommended; gaining control of any lumbar or accessory renal vessels encountered prior to performing the aortotomy helps avoid troublesome backbleeding. The superior aspect of the graft limb tunnels are then completed, taking care to maintain a course anterior to the common iliac vessels but posterior to the ureters. Between 5000 and 7000 units of heparin are then administered, with additional heparin given throughout the procedure to maintain the activated clotting time near the target range of 250 to 300 seconds. After allowing sufficient time for the heparin to circulate, atraumatic vascular clamps are placed above the IMA and just below the renal arteries. The distal clamp is applied first to avoid any distal embolization of plaque dislodged with placement of the proximal clamp. If an end-to-end anastomosis is planned, the aorta is transected 1 to 2 cm below the proximal clamp, and a short segment of the distal aortic cuff is excised (Fig. 21-4A). This results in better exposure of the aortic neck and a more precise proximal reconstruction, and also allows the graft to lie flat against the vertebral column rather than anteriorly oriented, facilitating later retroperitoneal coverage. If necessary, a thromboendarterectomy of the infrarenal neck is carried out at this point (Fig. 21-4B). Anastomosis is performed with a running suture of 3-0 polypropylene (Fig. 21-4C). The distal aorta is then oversewn with two layers of a running monofilament suture or stapled with a surgical stapler. If an end-to-side anastomosis is performed, an anterior longitudinal arteriotomy is carried out after placement of proximal and distal transaortic clamps. If necessary, an endarterectomy is performed and anastomosis carried out after the graft is beveled appropriately (Fig. 21-4D). If minimal plaque is present, the distal anastomosis is performed to the common femoral artery, and individual dissection of the superficial femoral and profunda femoral arteries is not necessary.
Another point of some debate concerns optimal management of patients with multilevel occlusive disease. The question frequently arises as to whether or under what circumstances a concomitant or staged outflow procedure should be performed. It is generally believed that up to 80% of patients with both inflow and outflow disease will be substantially improved following aortofemoral bypass grafting.11,22 Other reports, however, have suggested that as many as a quarter to a third of such patients will not have significant symptomatic relief with an inflow procedure alone.23 Although no single parameter exists to reliably guide the surgeon to know in which circumstances a combined procedure is optimal, severity of distal ischemia is probably the most important factor to be considered. Overall medical condition of patients and their ability to tolerate a prolonged operative procedure is also clearly important. Finally, the status of the profunda femoral artery must be taken into consideration. In the presence of SFA occlusion, a profunda that is atretic or extensively diseased may well be unable to provide sufficient collateral runoff to the foot.
If on the one hand, the bypass procedure is undertaken for claudication alone or mild rest pain, restoring adequate inflow may provide sufficient and relatively durable symptomatic relief. If on the other hand, significant tissue loss is present, a combined inflow and outflow procedure is likely warranted if limb salvage is to be achieved. If several operating teams are used, performing both procedures at the same time can be done in an acceptably timely fashion and has been found to be safe. Indeed, several recent reports found no significant differences in operative mortality or perioperative morbidity in patients undergoing concurrent inflow and outflow procedures compared with those having major inflow reconstruction alone.53,54 Although staged revascularization may be preferable in certain circumstances, both the risk of wound and graft infection resulting from redissection in the groin and the risk of progressive tissue loss during the initial recuperative period must be considered with this approach.
Results
Aortobifemoral bypass grafting is associated with patency rates that are among the highest reported for any major arterial reconstruction. As indicated earlier, 5-year primary patency rates of 70% to 88%29,31,32 and 10-year rates of 66% to 78%29 have been described. Better rates have been realized in those patients with good infrainguinal outflow operated on for claudication, compared with those with limb-threatening ischemia and associated infrainguinal occlusive disease. In general, patients with disease limited to the aortoiliac region have excellent relief of symptoms following aortobifemoral grafting, whereas those with multilevel disease have less complete levels of symptom diminution. Perioperative mortality rates average 4%, whereas 5-year survival rates between 70% and 75% have been reported.31,55,56 This latter rate is notably less than the 5-year survival rate of age-matched control population but on par with that typically seen for claudicants in general.
Although the early and late mortality rates are similar across different age groups, the 5-year primary and secondary patency rates are significantly increased with each increase in age group.33 Reed et al. reported that primary patency rates were 66%, 87%, and 96%, and secondary patency rates were 79%, 91%, and 98% (Fig. 21-5), respectively, for those younger than 50, 50 to 59, and older than 60 years of age.33 It seems prudent, based on these findings, to apply caution in the application of aortobifemoral bypass grafting for younger patients with virulent aortoiliac disease. The potential impact of graft failure and need for subsequent complex interventions should be considered, especially given the longer life expectancy of younger patients. Full utilization of all medical and endovascular options appears to be the best first-line option for younger patients with severe aortoiliac occlusive disease.
Extra-Anatomical Bypass
When comorbid disease renders a patient with aortoiliac occlusive disease particularly unsuitable for major vascular surgery and aortic cross-clamping, or when sepsis, prior surgery, or the presence of a stoma presents a hostile surgical environment for abdominal exploration, several alternatives are available to the vascular surgeon. Reconstructive options in which the thoracic aorta, axillary, iliac, or femoral arteries serve as donor vessels are generally referred to as extra-anatomical to distinguish them from the inline flow represented by an aortobifemoral procedure. The concept of extra-anatomical arterial reconstruction emerged in the 1950s during a time of many new developments in the field of vascular surgery. Freeman and Leeds provided one of the first descriptions in 1952 in their report of the use of the SFA as the conduit for a crossover femorofemoral bypass graft.57 These approaches are also called on in desperate situations represented by infection of a previously placed aortic graft.
Axillobifemoral Bypass
Axillobifemoral bypass grafting was introduced by Blaisdell58 in the early 1960s and has since enjoyed increasing popularity as an alternative to aortobifemoral bypass. This is largely due to the reliability of the axillary artery as a donor vessel and the minimal morbidity incurred, making it a particularly appealing option for patients with significant operative risk from comorbid disease. It is also appropriate in patients with significant aortoiliac occlusive disease of the distal aorta and the iliac arteries in the setting of intraabdominal sepsis, a history of multiple prior abdominal operations, intraabdominal adhesions, or prior pelvic irradiation. Of note, LoGerfo et al.59 have shown that axillobifemoral grafting has improved long-term patency compared with axillo-unifemoral grafting, presumably owing to the increased flow afforded by the second outflow limb.
The CFAs are then dissected through standard bilateral short groin incisions, and a second subcutaneous tunnel is fashioned between them in an extrafascial suprapubic plane. A Dacron or polytetrafluoroethylene (PTFE) graft, typically 8 mm in diameter, is then drawn through the tunnel. Although there is no convincing evidence that one graft material is superior to the other, several reports support the common practice of using an externally reinforced graft.60,61 Newer grafts are available that are prefigured in an axillobifemoral configuration, thereby reducing from four to three the number of anastomoses needed. As in aortobifemoral bypass grafting, unrestricted outflow should be ensured by carrying the hood of the femoral grafts down over the profunda orifice and performing an endarterectomy or profundaplasty when necessary. If a prefigured graft is unavailable, the origin of the cross-femoral graft can be tailored to the body habitus of the patient. In most cases, the graft is taken off the distal hood of the descending axillofemoral graft. In particularly obese individuals, however, it may be preferable to move the takeoff more proximally to prevent kinking at the level of the inguinal ligament. Orienting the takeoff of the crossover graft at an acute angle to give an S-shaped final configuration has been associated with higher patency rates in some studies.62
Many of the complications following axillofemoral grafting are directly related to the graft and potentially avoidable. Disruption of the proximal anastomosis, or axillary pullout syndrome, can be minimized by proper orientation of the proximal hood and ensuring that the descending limb of the graft is free from undue tension.63 Kinking and subsequent thrombosis of the graft can be reduced by strict attention to tunnel position and use of a reinforced conduit. Given the minimal physiological insult, most patients undergoing axillofemoral grafting are ambulatory and able to tolerate a regular diet on the first postoperative day.
Reported long-term patency rates of axillofemoral grafts have varied significantly, ranging from as low as 29% to as high as 85%.60,64–66 Favorable results were reported by Passman et al.,67 who achieved 5-year patency rates of 74% and a long-term limb salvage rate of 89%, and who are vocal advocates of a wider use for this approach. In general, axillobifemoral grafting should be reserved for high-risk patients with significant tissue loss and in danger of limb loss, and not be used for treating claudication.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree